Seabird Migration Strategies: Flight Budgets, Diel Activity Patterns, and Lunar Influence
- 1MARE – Marine and Environmental Sciences Centre, ISPA - Instituto Universitário, Lisbon, Portugal
- 2British Antarctic Survey, Natural Environment Research Council, Cambridge, United Kingdom
- 3BirdLife International, Cambridge, United Kingdom
- 4CESAM and Departamento de Biologia Animal, Faculdade de Ciências, Universidade de Lisboa, Lisbon, Portugal
- 5Department of Zoology, University of Cambridge, Cambridge, United Kingdom
- 6Centre d’Etudes Biologiques de Chizé (CEBC), UMR 7372 CNRS - Université de la Rochelle, Villiers-en-Bois, France
- 7School of Environmental Sciences, University of Liverpool, Liverpool, United Kingdom
- 8Department of Zoology, University of Oxford, Oxford, United Kingdom
- 9UMR 6249 Chrono-environnement, Université de Bourgogne Franche-Comté, Besançon, France
- 10Groupe de Recherche en Ecologie Arctique, Francheville, France
- 11Institut de Recerca de la Biodiversitat (IRBio) and Departament de Biologia Evolutiva, Ecologia i Ciències Ambientals, Universitat de Barcelona, Barcelona, Spain
- 12Norwegian Institute for Nature Research (NINA), FRAM – High North Research Centre for Climate and the Environment, Tromsø, Norway
- 13Wildlife Research Division, Environment and Climate Change Canada, Mount Pearl, NL, Canada
- 14UMR ENTROPIE, Université de La Réunion, IRD, CNRS, IFREMER, Université de Nouvelle-Calédonie, Université de La Réunion, La Réunion, France
- 15Institute of Ecology and Evolution, Friedrich Schiller University, Jena, Germany
- 16Department of Behavioral Ecology and Evolutionary Genetics, Max Planck Institute for Ornithology, Seewiesen, Germany
- 17Working Group Wildlife Research at the Clinic for Birds, Reptiles, Amphibians and Fish, Justus Liebig University Giessen, Giessen, Germany
- 18Norwegian Institute for Nature Research (NINA), Trondheim, Norway
- 19Department of Biology, Norwegian University of Science and Technology, Trondheim, Norway
- 20Psychology Department, Memorial University, St. John’s, NL, Canada
- 21Terres Australes et Antarctiques Françaises, La Réunion, France
- 22Auckland Museum, Auckland, New Zealand
- 23School of Biological Sciences, University of Auckland, Auckland, New Zealand
- 24CSIRO Oceans and Atmosphere, Hobart, TAS, Australia
- 25FitzPatrick Institute of African Ornithology, University of Cape Town, Rondebosch, South Africa
- 26National Institute of Water and Atmospheric Research Ltd., Christchurch, New Zealand
- 27Department of Bioscience, Aarhus University, Roskilde, Denmark
- 28Arctic Research Centre, Aarhus University, Aarhus C, Denmark
- 29National Institute of Water and Atmospheric Research Ltd., Wellington, New Zealand
- 30Wageningen Marine Research, Wageningen University and Research, IJmuiden, Netherlands
- 31Bureau Waardenburg, Culemborg, Netherlands
- 32Graduate School of Fisheries Sciences, Hokkaido University, Hakodate, Japan
- 33Organization for the Strategic Coordination of Research and Intellectual Properties, Meiji University, Tokyo, Japan
Every year, billions of birds undertake extensive migrations between breeding and non-breeding areas, facing challenges that require behavioural adjustments, particularly to flight timing and duration. Such adjustments in daily activity patterns and the influence of extrinsic factors (e.g., environmental conditions, moonlight) have received much more research attention in terrestrial than marine migrants. Taking advantage of the widespread deployment in recent decades of combined light-level geolocator-immersion loggers, we investigated diel organisation and influence of the moon on flight activities during the non-breeding season of 21 migrant seabird species from a wide taxonomic range (6 families, 3 orders). Migrant seabirds regularly stopped (to either feed or rest) during migration, unlike some terrestrial and wetland birds which fly non-stop. We found an overall increase for most seabird species in time in flight and, for several species, also in flight bout duration, during migration compared to when resident at the non-breeding grounds. Additionally, several nocturnal species spent more of the day in flight during migration than at non-breeding areas, and vice versa for diurnal species. Nocturnal time in flight tended to increase during full moon, both during migration and at the non-breeding grounds, depending on species. Our study provides an extensive overview of activity patterns of migrant seabirds, paving the way for further research on the underlying mechanisms and drivers.
Introduction
Every year, billions of birds migrate between breeding and non-breeding areas, often undertaking extensive journeys across unfavourable environments. Long crossings over seas, deserts or high mountain ranges are not uncommon in species as diverse as wildfowl, waders, passerines and raptors (Newton, 2008). Different environments impose different constraints, forcing migrants to respond accordingly. At one end of the spectrum are journeys across oceans by some terrestrial or wetland birds that cannot feed en route, and avoid landing on the water to rest, leading to long non-stop flights (e.g., bar-tailed godwits Limosa lapponica baueri can fly continuously for ∼10,000 km between Alaska and New Zealand; Gill et al., 2009). Slightly less constrained are birds crossing a region where they can stop to rest or to wait for better conditions, but in general cannot forage, such as the numerous passerines, raptors, and other species which migrate over the Sahara Desert (Klaassen et al., 2008; Strandberg et al., 2009; Lemke et al., 2013). Finally, birds sometimes have to fly over moderately challenging barriers, i.e., areas where they can stop to rest and even find some food, but that are not suitable for long periods of residency; this applies to non-tropical seabirds migrating over equatorial oceans where marine productivity and wind speeds are consistently low (Mann and Lazier, 2005). The extent to which migrating birds must adapt their daily routines when crossing unfavourable habitats should depend on the ratio of energy accumulated while feeding to energy spent on travel (Strandberg and Alerstam, 2007).
It might seem intuitive that birds should spend more time in flight during migration than at other times of year, particularly when foraging conditions are unfavourable, as is the case for many terrestrial and wetland birds (Newton, 2008). Flying seabirds, in contrast, mostly search for food while on the wing and regularly commute between ephemeral food patches (Weimerskirch, 2007). Consequently, they might maintain the same bearing towards their ultimate non-breeding destination, but forage en route and not necessarily increase flying time during migration. Additionally, as seabirds can make efficient use of predictable winds over large scales (Felicísimo et al., 2008; Weimerskirch et al., 2017), they may not need to greatly increase flying time during migration. As most seabirds can land on water anywhere, and at any time, with low risk of predation, they may show less propensity for long non-stop flights than other migratory birds. Although seabirds include some iconic long-distance migrants, such as the Arctic tern Sterna paradisaea, which migrates from Arctic breeding sites to spend the non-breeding season in the Southern Ocean (Egevang et al., 2010), we know relatively little about how these journeys are structured. However, there is evidence in just a few species of greater flight time during migration than in the rest of the non-breeding period (Mackley et al., 2010; Yamamoto et al., 2010; Dias et al., 2012a; Clay et al., 2017; Weimerskirch et al., 2020), and of longer flight bouts, although still only ca. 1–2 h (Mackley et al., 2011; Dias et al., 2012a; Clay et al., 2017).
The optimal time of the day to travel, feed, or rest can vary throughout the annual cycle depending on constraints mediated to some extent by light levels, which affect food detectability and availability, predation risk, wind conditions, and navigational cues. Seasonal and diel variation in such constraints affect the flight budgets of different species. Terrestrial bird species may either migrate exclusively at night (e.g., many passerines; Biebach et al., 2000), only during daylight (e.g., many raptors using thermal soaring; Strandberg et al., 2009), or use a combined strategy (e.g., when flying over ecological barriers; Alerstam, 2009; Adamík et al., 2016). Similarly, seabirds may concentrate most of their flight time during the day (e.g., breeding albatrosses, Phalan et al., 2007), be active both in daylight and darkness (e.g., white-chinned petrels Procellaria aequinoctialis, Mackley et al., 2011), or fly primarily at night (e.g., several petrels, Navarro et al., 2013; Dias et al., 2016; Rayner et al., 2016). Even within species, the degree of nocturnality can vary with environmental conditions (Dias et al., 2012b). Such flexibility suggests a high potential for adaptation of daily rhythms to the additional challenges faced during migration (e.g., crossing of unfavourable areas). A few studies have found evidence of seabirds becoming more nocturnal in their flight activity (Dias et al., 2012a; Hedd et al., 2012; Fayet et al., 2020), and that nocturnal flight bouts are longer during migration than in the non-breeding period (Mackley et al., 2011; Dias et al., 2012a; Clay et al., 2017). However, little is known about the extent to which most seabirds adjust their diurnal and nocturnal flight schedules while migrating, despite the useful insights it would provide into the challenges faced during this energetically demanding phase of the annual cycle.
Some of the differences in activity between day and night can be explained by light levels, and these are partially moderated by moonlight. A few studies based on landings of migrants or mist-netting suggest an influence of the lunar cycle on migratory intensity in terrestrial birds (Pyle et al., 1993; James et al., 2000; Speicher et al., 2011). However, the effect is well documented only in the European nightjar Caprimulgus europeaus, in which synchronous movements occurred 11 days after full moon - attributed to better foraging conditions during full moon and the time it takes to reach the ideal departure fuel load (Norevik et al., 2019). In seabirds, lunar influences on aerial activity have been mostly demonstrated in relation to foraging (Phalan et al., 2007; Yamamoto et al., 2008; Mackley et al., 2011; Dias et al., 2016; Pastor-Prieto et al., 2019), as moonlight can influence both prey availability (Regular et al., 2011) and prey detectability (Cruz et al., 2013). However, return to the colony of migrant Barau’s petrel Pterodroma baraui appeared to be regulated by moonlight (Pinet et al., 2011), and Cory’s shearwaters fly more and are more nocturnal on moonlit nights during migration (Dias et al., 2012a).
The increasing availability of tracking data in recent decades has provided opportunities to address fundamental questions relating to the scheduling of seabird flight activity and the effect of the moon. Two types of data are of particular interest and can be provided by the same small devices (light-level geolocators) throughout the non-breeding season: ambient light levels can be used for geolocation (providing longitude from the timing of local noon, and latitude from day length), and salt-water immersion can be used to determine timing and duration of flights (i.e., when loggers are dry). These loggers have been deployed widely on seabirds in the past 1–2 decades, shedding light on the migration patterns of many species (see Supplementary Table 1 for examples). Here, we take advantage of tracking and immersion data from 526 individuals of 21 species from six families to understand (1) how seabirds adapt their behaviour during migration, and in particular whether they change (a) their overall time in flight, and (b) ratios of total flight in daylight vs. darkness; and (2) whether moon illuminance is associated with flight activity during migration. We compare activity patterns during migration (here defined as the long-distance directed movements to and from wintering grounds), to those during the non-breeding period (here defined as the time spent resident in the main non-breeding areas), as during the latter, birds are less constrained than in other parts of the annual cycle (e.g., during breeding, when they have to return to colonies to incubate or provision their young; Phillips et al., 2017).
Materials and Methods
We collated geolocation (twice-daily positions at local midday and midnight) and salt-water immersion data (providing the timing and duration of flights and landings) for 21 migratory seabird species, collected using British Antarctic Survey (Cambridge, United Kingdom), Biotrack (Wareham, United Kingdom) or Intigeo (Cambridge, United Kingdom) loggers deployed on adults at 27 different colonies. For original papers detailing fieldwork and processing methods, and number of individuals, year, and moon phase, see Supplementary Tables 1, 2. We focused on pelagic species for which these data were available year-round. Other taxa were excluded because they are often terrestrial during the non-breeding season (e.g., most gulls), or are pelagic but rest on floating objects - including ice - at sea [terns, Antarctic petrel Thalassoica antarctica (Delord et al., 2020) and giant petrels Macronectes spp.], or hold one leg out the water while resting (some alcids, Fayet et al., 2016), all of which affect apparent flight duration as measured using immersion loggers. In addition, analyses were restricted to long-distance migrants in which there were clear migration phases that could be separated from time spent in the non-breeding area, and for which the spatial error associated with geolocation (around 190 km; Phillips et al., 2004) was not a major issue. At least part of the migration phases also had to occur outside of the period around the equinoxes when latitude is difficult or impossible to estimate from light data (Hill, 1994). Procellariidae (shearwaters and petrels) are therefore better represented in our dataset than other families.
Definition of Migratory Periods
Migratory periods were defined for each bird based on dates provided by data owners, corresponding to those when the bird (1) left the breeding area, (2) arrived at the non-breeding grounds, (3) left the non-breeding grounds, and (4) returned to the breeding area. If these dates were not provided, we identified them manually for each individual using the time series of longitude, latitude or distance from the breeding area, associating approximately constant values of all these variables to non-breeding areas. Only data from the migration and non-breeding periods were included in analyses. Time series of distance to the breeding colony, colour-coded by migratory periods, are available in Supplementary Materials (Supplementary Figure 1).
Definition of Daylight vs. Darkness and Moon Parameters
For each bird location (available twice-daily), we obtained the timings of civil twilights (times when the sun is 6° below the horizon) using the crepuscule function in the package maptools (Bivand and Lewin-Koh, 2018) in R (R Core Team, 2019) and used these values to separate activity into daylight (time between civil dawn and civil dusk) and darkness (time between civil dusk and civil dawn). For each bird location, we also calculated the illuminated fraction of the moon using the moonAngle function in the oce R package (Kelley and Richards, 2021). We then grouped values of illuminated fraction of the moon into three phases (new moon: between none and a third of the moon illuminated, quarters: between a third and two thirds of the moon illuminated, and full moon: between two thirds and all of the moon illuminated, similar to Dias et al., 2012a). No daylight-darkness or lunar phase values could be calculated around equinoxes as estimated latitudes during this period are unreliable; therefore, corresponding data were removed from the analyses.
Flight Budgets
We determined flight patterns from salt-water immersion data. For most species, the loggers tested for immersion at 3-s intervals and recorded the sum of positive tests (a value x from 0 to 200) at the end of each 10 min-period. The exception were the loggers used on Leach’s storm petrels Oceanodroma leucorhoa, which sampled for immersion every 30 s but also binned the data into 10 min intervals, and the loggers used on Cory’s shearwaters, which recorded the timing of every change of state from wet to dry, or vice versa, of ≥6 s. We considered time on the water to be the percentage of time in each 10 min bin in which the logger was wet, i.e., x/200. Similarly, flight time was calculated as the percentage of time in each 10 min bin in which the logger was dry, i.e., (200−x)/200. We then calculated the mean flight time during daylight and darkness for each individual. We considered flight bouts as any continuous sequence of one or more 10 min intervals in which the logger was fully dry, following Phalan et al. (2007). As flight bouts are included regardless of their duration (min. 10 min, as this is the resolution of the data), this approach did not allow us to distinguish between commuting flight during migration, and flight associated with foraging.
Night Flight Index
We calculated a Night Flight Index (NFI; an index of nocturnal flight activity) for each day (consecutive daylight and darkness period) following Dias et al. (2012a):
where %FN = % of darkness time spent in flight, and %FD = % of daylight time in flight. Percentages of time were used instead of absolute values in order to control for the effect of changing photoperiod at different latitudes during the year. This index varies between −1 when all the flight activity each day occurs in daylight (hereafter called “diurnal” behaviour), and 1 when all the flight activity takes place in darkness (hereafter called “nocturnal” behaviour). We mapped this index in space by calculating daily locations for each individual (average latitudes and longitudes of the two daily locations) and associating these with the daily NFI values. These spatially referenced individual daily locations were then overlaid in an Icosahedral Snyder Equal Area Aperture 3 Hexagon Discrete Global Grid (resolution 6), and values averaged per grid cell.
Statistical Analyses
We tested whether activity patterns differed during periods spent on migration and in the non-breeding grounds, or during the different phases of the lunar cycle, by modelling daily activity metrics separately for each species using Linear Mixed Models (LMMs) using the R package nlme (Pinheiro et al., 2021), with individual and time block within individual as random effects. Time blocks correspond to consecutive days of the same breeding stage and moon phase, and were included in the analysis to control for temporal autocorrelation. We analysed the following response variables: (1) % of time in flight, (2) flight bout durations, and (3) NFI. For ease of interpretation, we split our dataset into new moon, moon quarter and full moon, and into daylight and darkness [for (1) and (2) only]. We then tested the effect of the migratory phase (with the non-breeding period as a reference) separately for each sub-dataset. Similarly, we split our dataset into outward or return migration, and the non-breeding period, and between darkness and daylight [for (1) and (2) only]. We then tested separately the effect of moon phase (with new moon as a reference) for each sub-dataset. Also for ease of interpretation, we contrasted only new moon and full moon phases to test for the effect of moon phase. Variables were transformed as follows before fitting the models: proportion of time in flight was logit-transformed, flight bout duration was log-transformed, and the NFI was rescaled between 0 and 1 [i.e., (NFI + 1)/2] and logit-transformed. Unless stated otherwise, we obtained single values per species (e.g., percentage of flight time over a certain migratory period, or a certain moon phase) by averaging values across days (separating daylight and darkness, where appropriate) for each individual, and then we used the mean of those values in posterior analyses. The percentage of time spent in flight and the NFI each day during migration (outward and return migrations separately) in relation to the percentage of time in flight during the non-breeding period was compared between transequatorial and non-transequatorial migrant species (classified according to the movements of the majority of individuals, see Supplementary Table 2 for details).
All calculations of flight budgets, mapping and analyses were carried out using the R statistical software.
Results
General Flight Activity Patterns
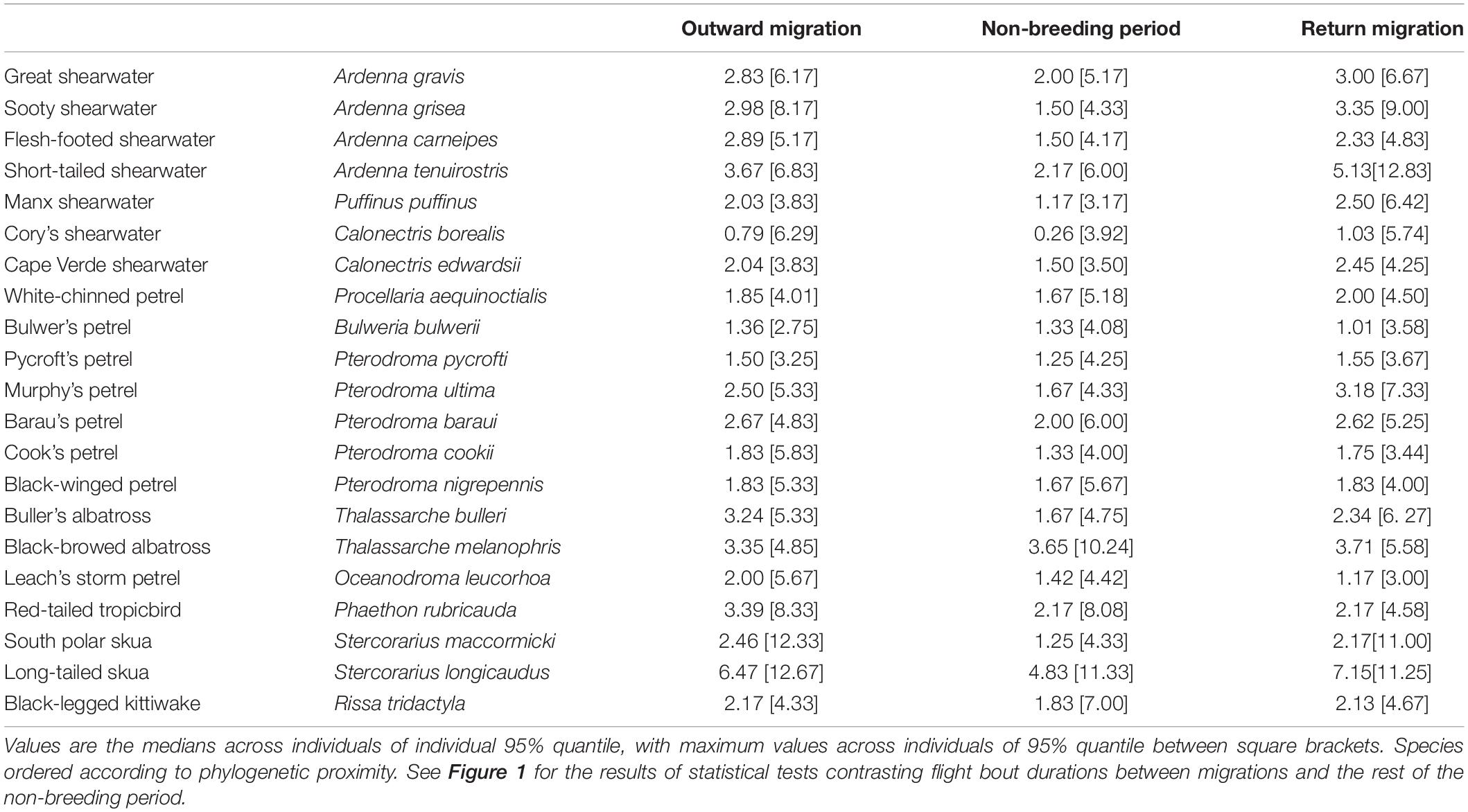
All species spent more time in flight and had longer flight bouts during at least one migration period (outward, return, or both) than in their non-breeding areas (Tables 1, 2, Figures 1, 2A–C, Supplementary Figure 2). This increased flight time occurred during both daylight and darkness (Figure 1), with the magnitude of the change varying markedly among species (Figure 2C). The ratio of flight time during migration to flight time during the non-breeding period varied from 1.16 in black-legged kittiwakes Rissa tridactyla to 4.63 in short-tailed shearwaters Ardenna tenuirostris (based on mean values, Supplementary Table 3 and Figure 2C). For reference, Supplementary Table 3 also provides latitudinal migratory range and migratory distance (here defined as distance between breeding and non-breeding range) for each species. Median flight bout duration rarely exceeded a few hours (Table 2). However, a few individuals of some species (great shearwaters Ardenna gravis, short-tailed shearwaters, red-tailed tropicbirds Phaethon rubricauda, south polar skuas Stercorarius maccormicki, long-tailed skuas Stercorarius longicaudus and black-legged kittiwakes) performed bouts >24 h, mostly during migration (Supplementary Table 4).
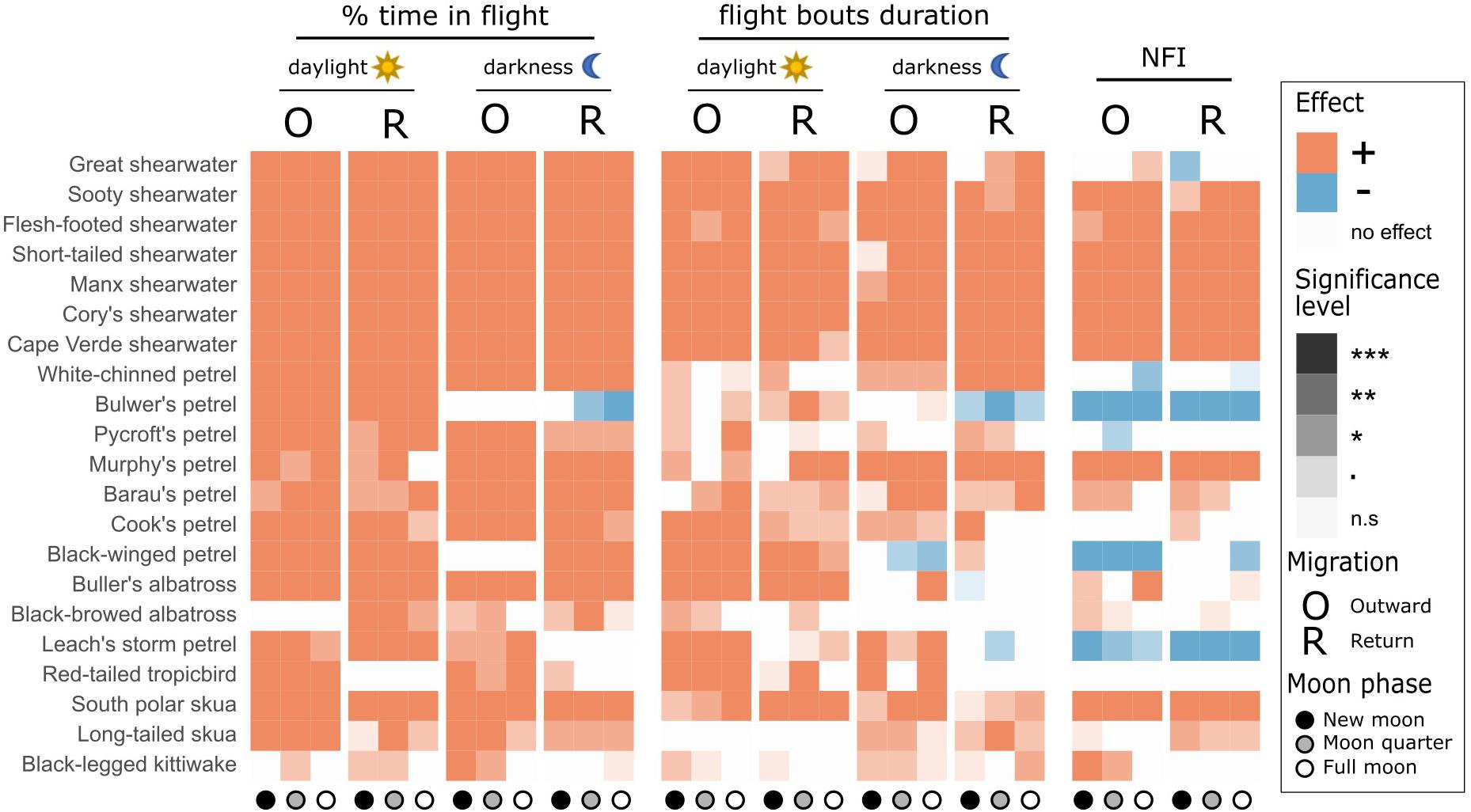
Figure 1. Representation of results of LMMs testing the effect of outward (O) and return (R) migration compared with the non-breeding period on three behavioural response variables: % of time in flight, flight bout duration, and the night flight index (NFI). Orange squares indicate that the value was significantly higher during the migratory stage than during the non-breeding period, blue squares that the value was significantly higher during the non-breeding period, and white squares that there was no significant difference. Shade represents level of significance. Effects are tested separately by moon phase (new moon: dark circles, moon quarters: grey circles, full moon: white circles) and for daylight vs. darkness, as appropriate. For scientific names, see Table 1. Significance levels: “***” = p-value < 0.001, “**” = p-value < 0.01, “*” = p-value < 0.05, “.” = p-value < 0.1, “n.s” = non-significant.
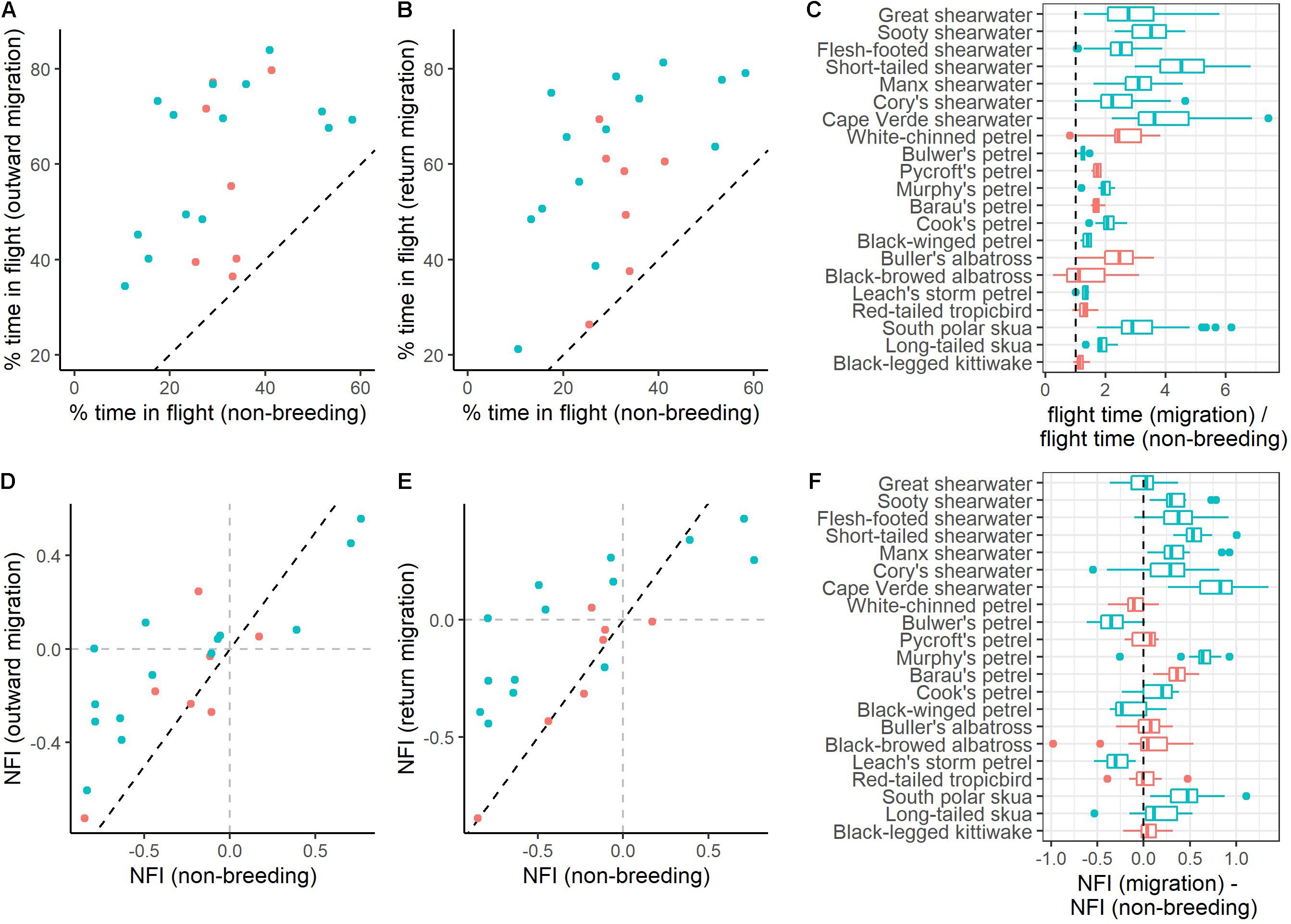
Figure 2. Behavioural adaptations to migration, contrasted between transequatorial (blue) and other (pink) migrants. (A,B) Relationship between flight time during migration and the non-breeding period, for outward and return migration, respectively. (C) Ratio of % flight time during migration over % of flight time during the non-breeding period (boxplots of mean values for each individual across seasons). Species above the dotted line in panels (A,B) or individuals right of the dotted line in panel (C) spend more time in flight during migration than during the non-breeding period. (D,E) Relationship between nocturnality levels during migration and winter. (F) Difference between NFI during migration and winter (boxplots of mean values for each individual across seasons). Species above the dotted line in panels (D,E) or individuals right of the dotted line in panel (F) are more nocturnal during migration than during the non-breeding period. (A,D) outward migration; (B,E) return migration.
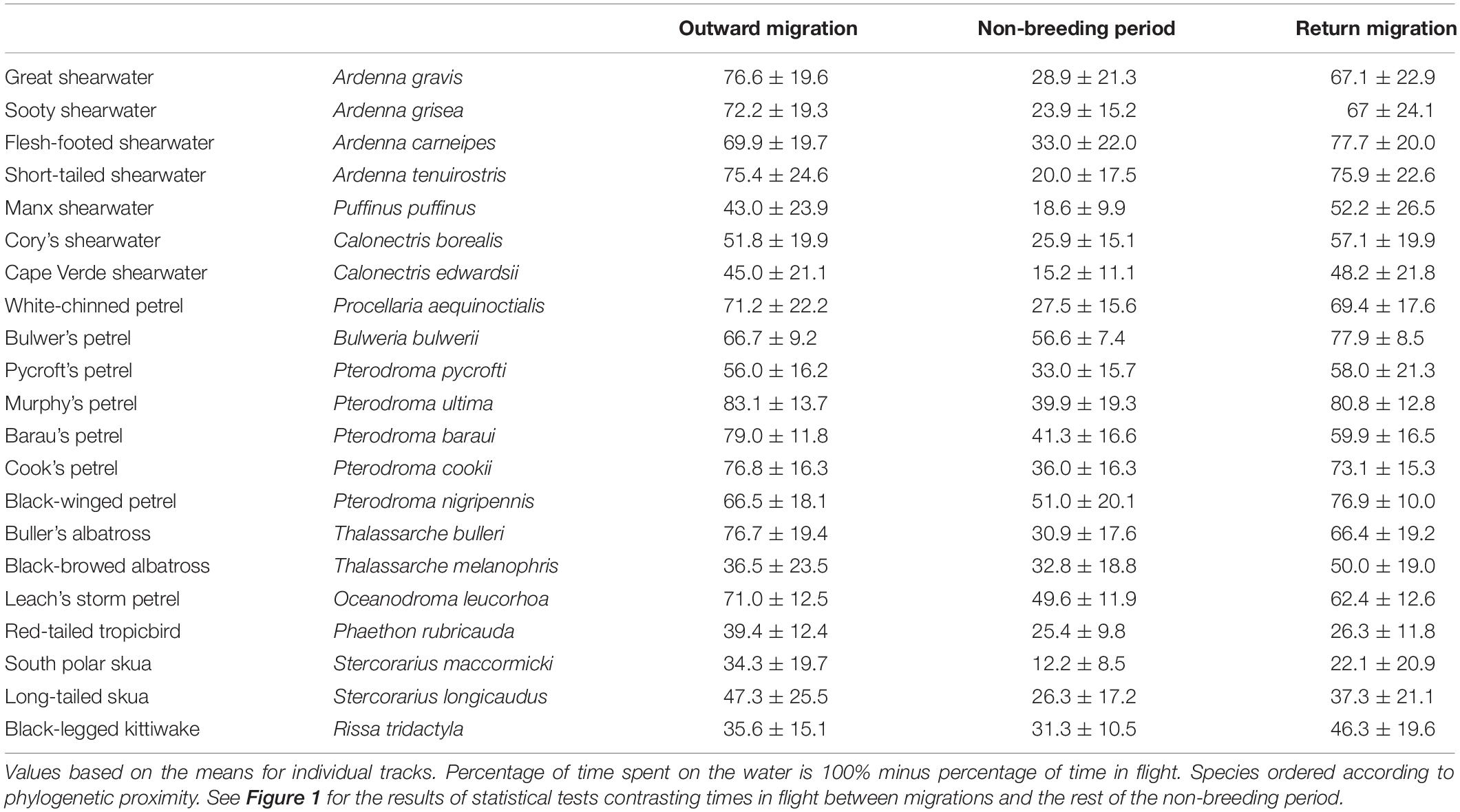
Table 1. Mean (±SD) percentage of time spent in flight during each migratory stage for different seabird species.
Despite the higher flight activity, most species spent a considerable amount of time on the water during migration (from 17% in Murphy’s petrel Pterodroma ultima during outward migration to 78% in south polar skua during return migration) (Table 1). In many species, a high percentage of time on the water during migration occurred in bouts >1 h [median across species of 40.0% (outward)/464% (return), with minima of 15.8% (outward)/7.5% (return) for Bulwer’s petrel Bulweria bulwerii and maxima of 78.3% (outward)/84.6% (return) for black-browed albatrosses Thalassarche melanophris; Supplementary Table 5].
During the three consecutive days with the greatest flight activity (hereafter termed the “peak migration period”), many species spent 80–90% of the time in flight, indicating they nevertheless rested for at least 2 h per day (Figure 3). However, there were several species that spent substantially less time in flight: for example, the proportions of time spent in flight in these three days during the outward and return migrations (median values across individuals) were only 67 and 65% in Cape Verde shearwaters Calonectris edwardsii, 53 and 65% in the mostly diurnal, black-legged kittiwakes, and 57 and 46% in red-tailed tropicbirds, respectively. Even when crossing equatorial regions, species often spent less than 75% of their time in flight, although this varied among transequatorial migrants: skuas often spent <50%, whereas Puffinus and Ardenna shearwaters spent most time in flight (Supplementary Figure 3).
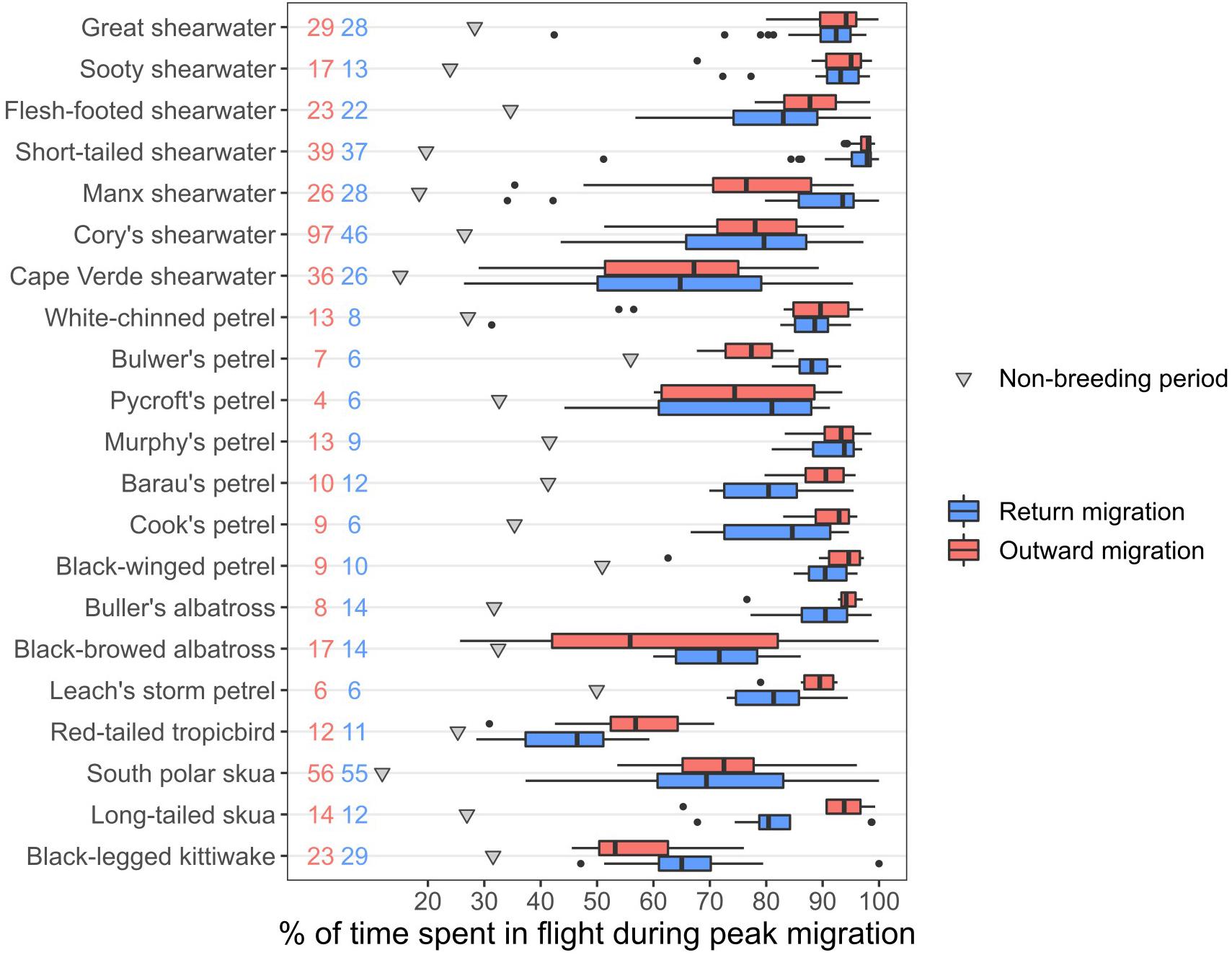
Figure 3. Percentage of time spent in flight during the three consecutive days with the highest flight activity for each individual, aggregated by species. Pink: outward migration, blue: return migration, grey triangles: average flight time during the non-breeding period, for reference. Black dots represent outliers of the boxplots. Numbers represent sample sizes (pink: outward migration, blue: return migration).
Daily Activity Budgets During Migration
During both migration and the non-breeding period, all species flew during both daylight and darkness, although there was a large variation in baseline (non-breeding period) NFI values among species (Figures 4, 5 and Supplementary Table 6). The most diurnal species during the non-breeding period was the black-legged kittiwake (baseline NFI: −0.8 ± 0.3), followed by the south polar skua (−0.8 ± 0.4) and most shearwaters, and then the two albatross species (−0.4 ± 0.5 and −0.2 ± 0.6). Only four species were predominantly nocturnal during the non-breeding period: Bulwer’s petrel (0.8 ± 0.2), black-winged petrel Pterodroma nigripennis (0.4 ± 0.3), Leach’s storm-petrel (0.7 ± 0.2) and to a lesser extent, white-chinned petrel (0.2 ± 0.6). For several species – both diurnal (e.g., the two skuas) and nocturnal (e.g., Bulwer’s petrel) – mean flight bout duration was longer in darkness than in daylight [comparison between individual daily means of flight bout duration in darkness vs. in daylight, paired t-test, all species combined: mean difference (in hours): 0.036, t = 7.779, df = 45181, p < 0.001; Supplementary Figure 4].
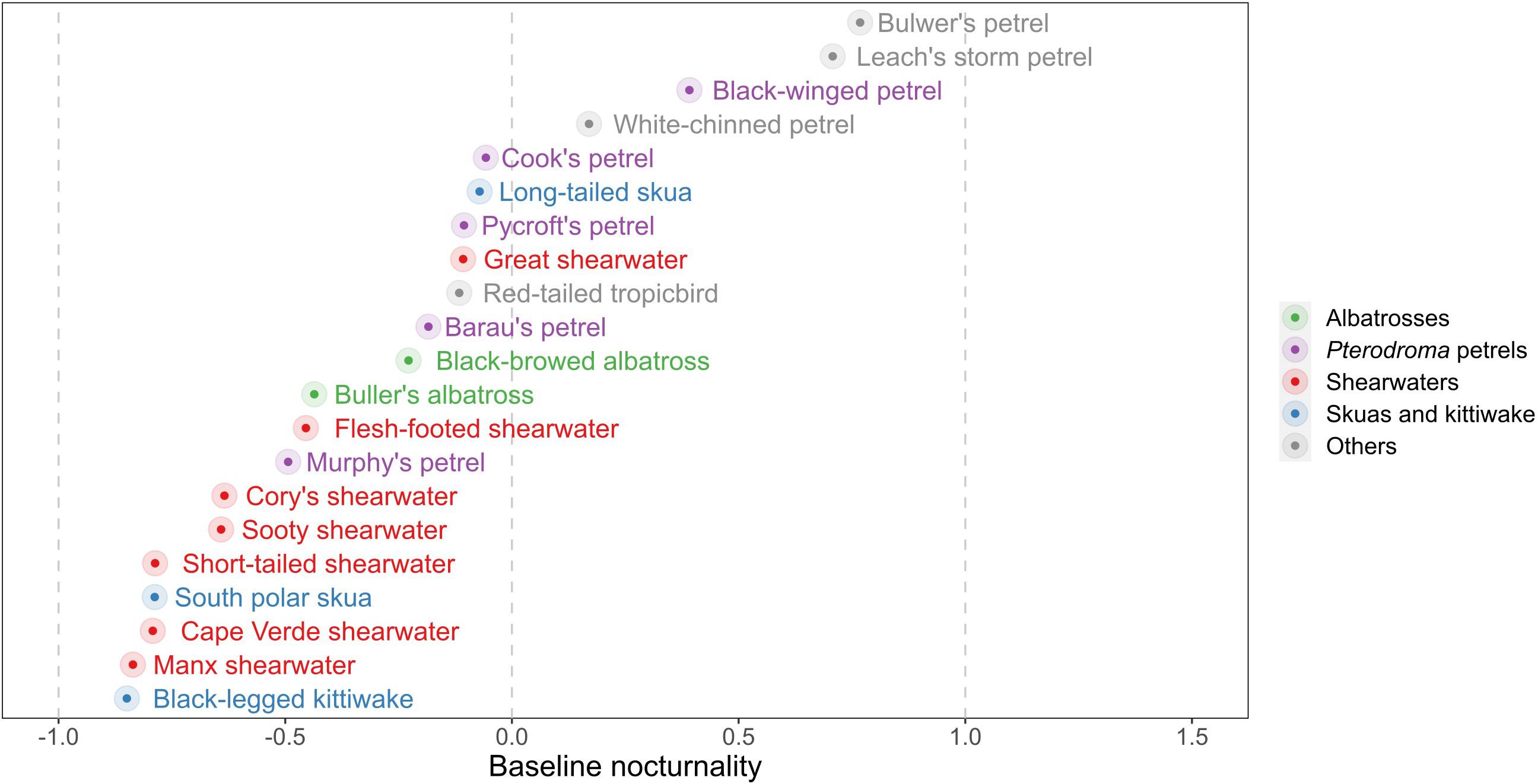
Figure 4. Night flight index during the non-breeding period averaged across days and individuals (coloured dots). Negative values correspond to diurnal species (i.e., species which fly more during daylight than darkness), while positive values are for nocturnal species (i.e., species which fly more during darkness than daylight). For exact values and standard deviations, see Supplementary Table 5.
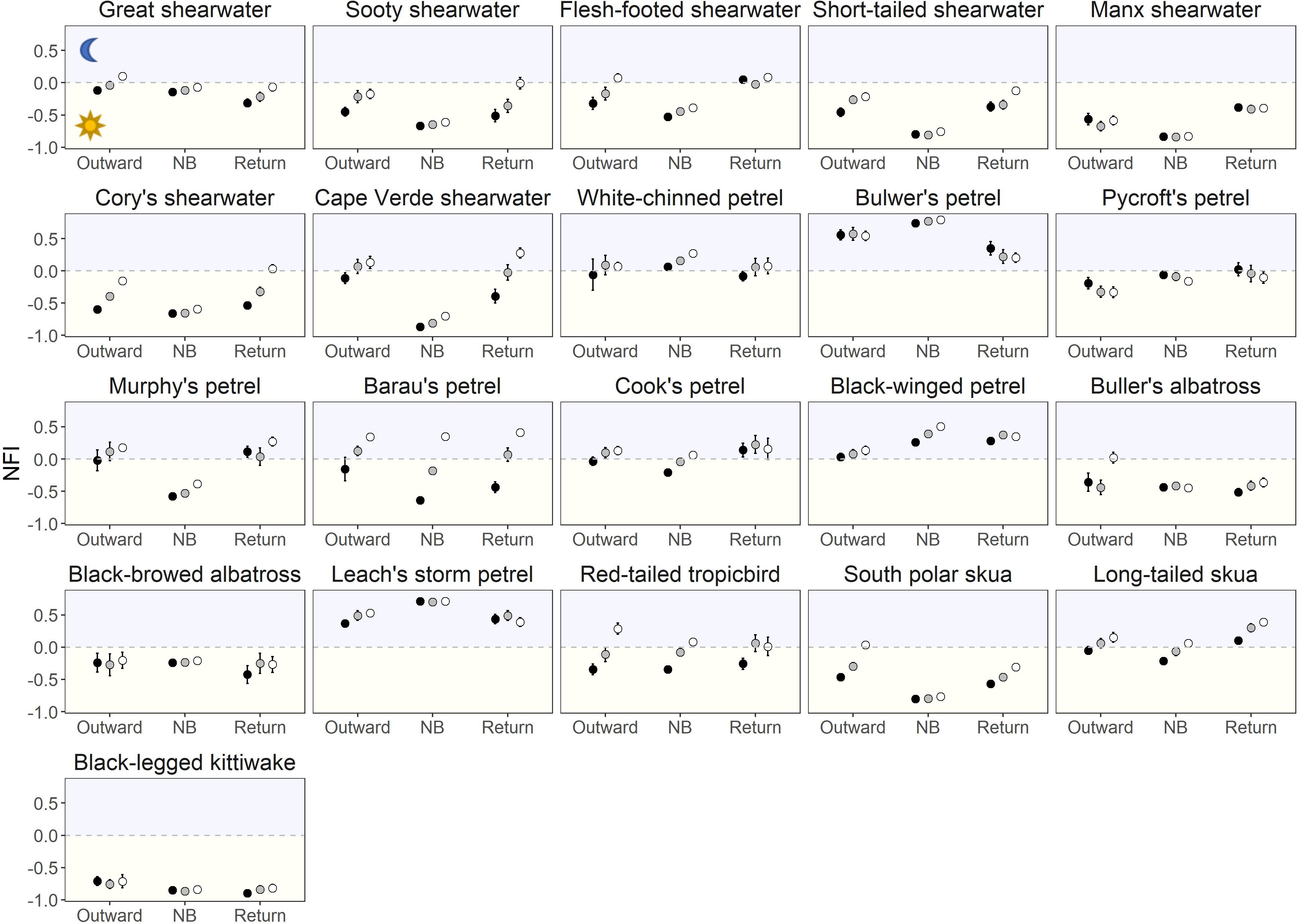
Figure 5. Effect of migratory stages (outward migration, NB: non-breeding period, return migration) and the moon (new moon: black dots, moon quarters: grey dots, full moon: white dots) on the night flight index (NFI). Results are presented as mean ± 95% CI around the mean. The levels of significance of the differences are shown in Figures 1, 6. For scientific names, see Table 1.
Most species were more nocturnal (i.e., increased NFI, mostly through a greater increase in time in flight during darkness than daylight) in the migration than non-breeding periods, but there were exceptions (Figures 1, 2D–F). In particular, the four species that were predominantly nocturnal at their non-breeding grounds (Bulwer’s petrel, black-winged petrel, Leach’s storm petrel, and white-chinned petrel) showed a reverse trend and became more diurnal during migration (i.e., there was a greater increase in time in flight during daylight than darkness; Figure 1). NFI changed little in other species, including those that were not clearly more diurnal or more nocturnal at their non-breeding grounds (i.e., cathemeral), but also a few that were clearly diurnal, such as albatrosses (Figures 1, 4). Transequatorial migrants appeared to be more diurnal at higher latitudes (where most species spent the non-breeding period), and more nocturnal in equatorial regions (Figure 6). This pattern was consistent across several families. However, in certain regions such as the Indian Ocean, some species appeared more diurnal around the tropics (Figure 6), which might suggest the presence of pelagic prey communities of a different nature (i.e., with more epi-pelagic and fewer mesopelagic prey), but more research will be needed to confirm this speculation.
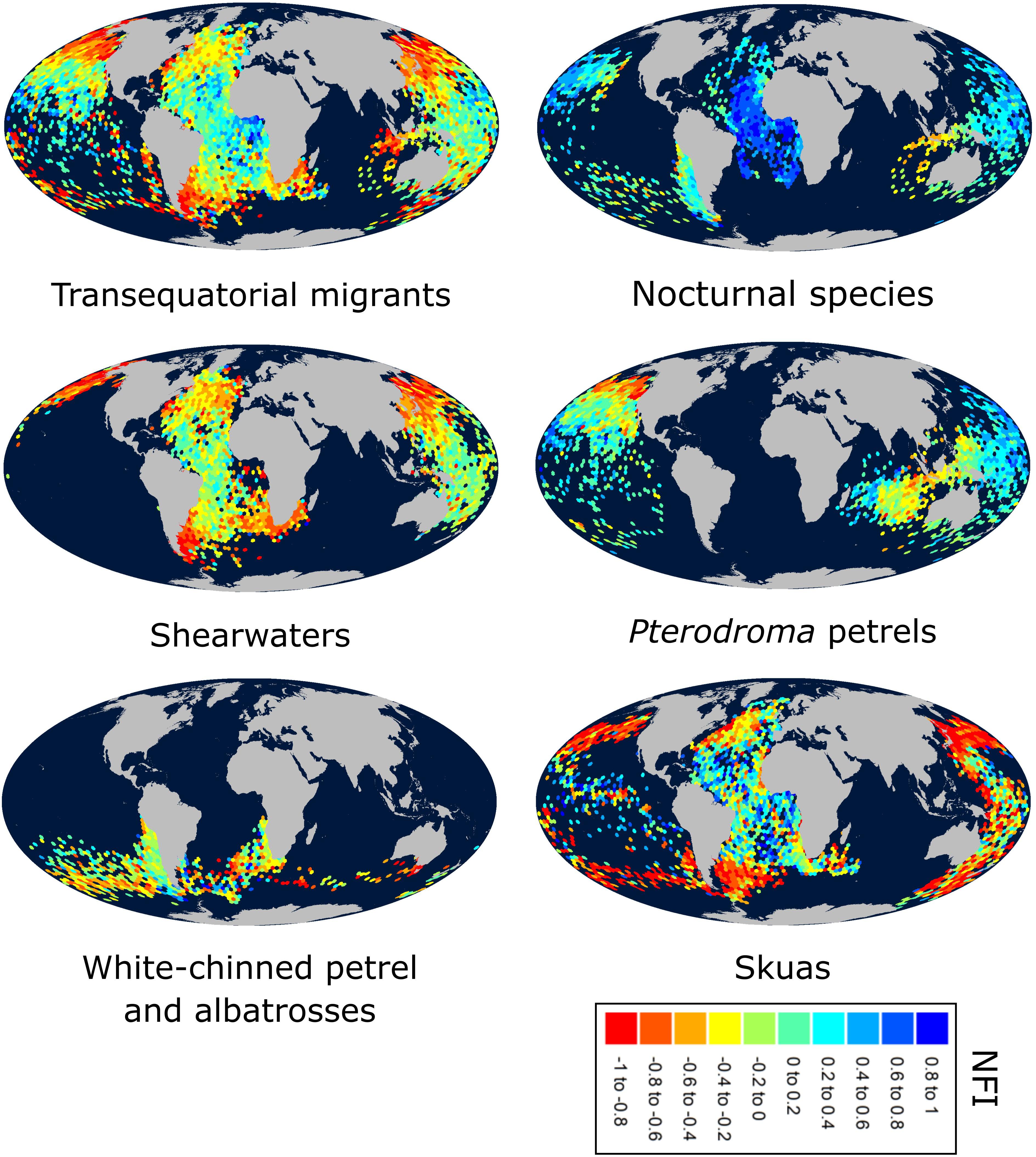
Figure 6. Map of the night flight index (NFI) throughout the non-breeding season for different seabird groups. “Transequatorial” species: sooty shearwater, flesh-footed shearwater, Manx shearwater, great shearwater, short-tailed shearwater, Cape Verde shearwater, Cory’s shearwater, Leach’s storm petrel, Bulwer’s petrel, Cook’s petrel, black-winged petrel, Murphy’s petrel, long-tailed skua, south polar skua; “Nocturnal” species (generally positive NFI during the non-breeding period – see Supplementary Table 6): Bulwer’s petrel, black-winged petrel, white-chinned petrel, Leach’s storm petrel.
Influence of Moon Phases
For many species there was increased nocturnal activity with increased moonlight in at least one period (during migration and/or when in non-breeding areas), with the exception of the Manx shearwater Puffinus puffinus, Pycroft’s petrel Pterodroma pycrofti, and Leach’s storm petrel (Figure 7). The mostly nocturnal Bulwer’s petrel showed different responses to the moon across the year: more nocturnal in full moon in the non-breeding areas, and the reverse during return migration (Figure 7 and Supplementary Figure 5). Several species that increased their NFI during full moon also reduced their activity in daylight (Figure 7 and Supplementary Figure 6B). This compensation was clear during migration, but was not apparent in non-breeding areas (Supplementary Figure 6B; paired t-test across species comparing the moon effect during daylight in the non-breeding areas vs. during migration: mean difference: −2.54, t = −2.33, df = 20, p = 0.031). There was no indication that the effect of moon phase on the NFI was more pronounced during migration (Supplementary Figure 6A; paired t-test across species: mean difference: 0.06, t = 1.67, df = 20, p = 0.110). Lunar phases had a similar effect on predominantly nocturnal and predominantly diurnal species (Supplementary Figure 6A; small effect during migration, no effect during the non-breeding period, Supplementary Table 7).
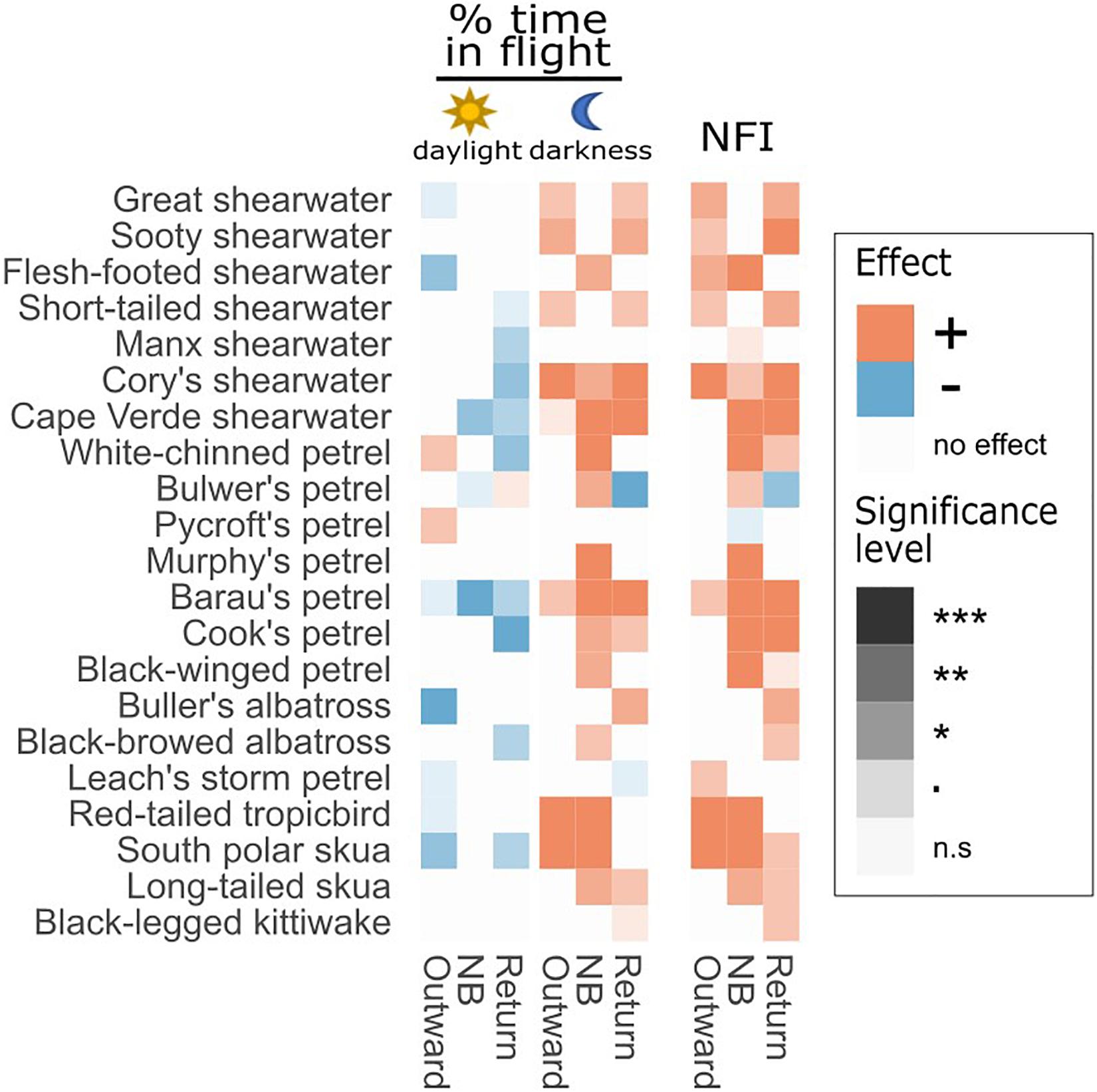
Figure 7. Behavioural changes during full moon compared with new moon. Colours and shades represent the results of LMMs testing the effect of the moon phase on two response variables: % of time in flight and the night flight index (NFI). Orange squares indicate that the value was significantly higher during the full moon than during the new moon period, blue squares represents the opposite effect and white squares that there was no significant difference. Effects are tested separately among stages of the non-breeding season (outward migration, NB: non-breeding period, return migration) and daylight vs. darkness, as appropriate. Significance levels: “***” = p-value < 0.001, “**” = p-value < 0.01, “*” = p-value < 0.05, “.” = p-value < 0.1, “n.s” = non-significant.
Discussion
Using an extensive dataset on movements and flight behaviour of 21 species from six families, we show how flying seabirds adapt their flight budgets to migration, and the influence of the moon. In general, time in flight, and in several species also flight bout duration, increased during migration, but the extent varied among species. Nevertheless, all species spent some time resting, even on the three days when flight activity peaked. Virtually all species adjusted their diel activity schedules during migration. We found some influence of the lunar phase on flight activity, but that was not necessarily more pronounced during migration than when birds were resident in their non-breeding areas.
Our study had broad taxonomic scope, yet we found a consistent increase in daily flight time during migration across species (and most pronounced among shearwaters) often two to three times higher than observed during the non-breeding period. This increase in flight time during migration suggests that all the study species were motivated to move faster over certain environments, possibly because these are less favourable habitats for foraging. This observed increase is consistent with results from species not studied here: the wedge-tailed shearwater Ardenna pacifica (Weimerskirch et al., 2020) and, to a lesser extent, several albatross species (Mackley et al., 2010; Gutowsky et al., 2014). For some species (e.g., most shearwaters), we also found an increase in flight bout duration during migration, which has also been observed in albatrosses (Mackley et al., 2010). Hence, our study shows that during migration, seabirds do not simply retain the same routine (for example, adjusting flight direction towards the preferred non-breeding region), but instead that they routinely change their flight budgets. Note that birds may fly less when resident at non-breeding grounds because of primary moult impairing flight capabilities (Tucker, 1991; Bridge, 2003; Cherel et al., 2016). However, the many gaps in knowledge of the timing of moult in seabirds (Bridge, 2007) prevent us from clearly attributing reduced flight activity to moult. In any case, many birds, including seabirds, avoid moulting during migration (e.g., Ramos et al., 2009), which may be a strategic choice in order to maintain high flight capability.
Our study provided a quantitative analysis of nocturnality, in the form of the Night Flight Index, which could be compared readily among species and periods. Most species that were diurnal in their non-breeding areas (i.e., when foraging without central-place or migration constraints) were also diurnal during both the outward and return migrations, and the opposite was true for species that were nocturnal in their non-breeding areas. However, regardless of the degree of nocturnality in the non-breeding period, the flight bouts of most species were longer in darkness than daylight throughout the time away from the colony, suggesting that night is preferred for (long-distance) travel. Many species re-adjusted the degree of nocturnality during migration (flying more at night than in the rest of the non-breeding period). Species that were nocturnal in their non-breeding areas (Figure 4) showed the opposite pattern (Figures 1, 2). Although not tested formally, this general pattern was also apparent in diurnal albatrosses (Mackley et al., 2010), wedge-tailed shearwaters (Weimerskirch et al., 2020) and Manx shearwaters (Fayet et al., 2020), and in the nocturnal Bugio petrel Pterodroma deserta (Ramírez et al., 2013). This suggests that seabirds adjust their flight time during the time of day when there is more scope for additional flight, which minimises the time spent travelling during the rest of the 24-h period when foraging is more profitable. The changes in nocturnality and the increase in overall flying time appeared particularly pronounced for non-tropical species migrating across the equator (e.g., all shearwaters except the great shearwater, as well as both skuas, Figures 2, 6). Although phylogeny and transequatorial migration were correlated in our sample, limiting the possibility of robust contrasts, this suggests that for non-tropical seabirds, equatorial waters act as a barrier that requires greater behavioural adjustments than crossing temperate or polar waters. This could be because equatorial waters are oligotrophic (Mann and Lazier, 2005), or because lower winds (i.e., doldrums; Lamb, 1975) make flight more challenging.
Although our results show that the studied seabirds increase their flight time during migration, the change is much less pronounced than in some other birds, such as certain waders and passerines (Newton, 2008). Even during the three consecutive migratory days with the greatest time in flight, many seabirds still spent a large proportion of time on the water (Figure 3). In addition, predominantly nocturnal species increased diurnal flight activity before allocating the entirety of the remaining time available for flying at night, and the converse applied to predominantly diurnal birds (daylight or darkness time never filled 100% with flight, Supplementary Figure 5). This suggests that most seabirds avoid spending too much time flying (which may be costly), and prefer to stop regularly, even during migration (Landers et al., 2011; Mackley et al., 2011; Gutowsky et al., 2014; Berg et al., 2019; Weimerskirch et al., 2020, but see van Bemmelen et al., 2019). However, it is difficult to investigate directly the energetic implications of these different behaviours, creating an avenue for future research. Finally, compared with passerines or waders crossing unfavourable terrestrial or marine environments (Newton, 2008), our study species did not undertake very long flight bouts (rarely more than a few hours; Table 2). This could suggest that long flight bouts would be very costly, although it is unlikely for soaring or gliding birds (Weimerskirch et al., 2000; Sakamoto et al., 2013), except in areas with particularly weak winds. Alternatively, long flight bouts could be unnecessary if food supply is adequate and weather conditions relatively benign, providing little incentive to proceed rapidly to non-breeding grounds.
Further evidence that seabirds are unwilling to greatly increase flight effort per day is the compensation observed in some species during full moon: birds that increased flight time during darkness, flew less during daylight. This suggests that when conditions allow, seabirds prefer to spread their flight effort over a wider period including darkness. Furthermore, even when crossing the equator, non-tropical seabirds spent a considerable amount of time on the water (Supplementary Figure 3), indicating benefits of stopping to rest (potentially because equatorial winds make flight more challenging), rehydrate, or refuel regularly during migration, even if foraging conditions might be suboptimal. Note that terrestrial and wetland species preparing for long migrations accumulate a large amount of fat (Schaub and Jenni, 2000; Krapu et al., 2006), even sometimes shrinking organs not used for migration (Piersma et al., 1999). However, this behaviour is likely to be costly, so if it can be avoided by seabirds by simply stopping en route to feed and rest, they may be less challenged by migration.
Our approach did not allow us to discern exactly what birds are doing when they stop flying, but the periods when all study species alternated between very short flights and landings suggests regular foraging during migration (Dias et al., 2012a). This is likely to include time spent digesting prey while sitting on the water (Jackson, 1988; Ropert-Coudert et al., 2004). However, the existence of long wet bouts during migration (Supplementary Table 5) suggests that birds might also spend time resting between flight bouts geared towards long-distance travel. Taxa other than seabirds also halt their migratory journeys to rest, even when no food is available. For instance, Brent geese Branta bernicla hrota leave Iceland in spring with enough fuel to cross the Greenland ice cap but nevertheless stop regularly to rest while en route at sites with no food available (Gudmundsson et al., 1995). There is evidence that long migratory flights can lead to muscle damage (e.g., in western sandpiper Calidris mauri and bar-tailed godwits, Guglielmo et al., 2001), or oxidative stress (European robin Erithacus rubecula, Jenni-Eiermann et al., 2014). Finally, sleep might be important. Although white-crowned sparrows Zonotrichia leucophrys gambelii can withstand sleep deprivation during migration (Rattenborg et al., 2004), and great frigatebirds Fregata minor can even sleep while on the wing (Rattenborg et al., 2016), it is unknown how ecologically relevant, widespread, and costly, these adaptations are. Many birds apparently need to sleep once they finish long migratory journeys (Schwilch et al., 2002; Németh, 2009; Covino and Cooney, 2015). Seabirds might therefore spend much of their time sleeping between flights, even during migration, to avoid the costs of sleep deprivation.
Almost all species showed increased flight activity during darkness, and increased nocturnality during the full moon for at least part of the year (Figure 7), but it is difficult to determine whether the lunar cycle has a greater influence on travel or foraging. For at least partly nocturnal foragers, intensified foraging effort on nights with a full moon may indicate they have to work harder as conditions are unfavourable, for example if prey performing diel vertical migration are less accessible, or conversely that foraging conditions are favourable (e.g., because of increased visibility) and that birds expend more effort because the returns are good (Mackley et al., 2011; Pinet et al., 2011; Cruz et al., 2013; Rubolini et al., 2015; Dias et al., 2016; Ravache et al., 2020). Such lunar influences on foraging activity, sometimes mediated by predator avoidance, have been reported in a wide range of taxa (Yamamoto and Trathan, 2015): not only in birds (Clarke, 1983; Evens et al., 2020), but also in insects (Kerfoot, 1967) and mammals (Fernandez-Duque, 2003; Penteriani et al., 2013). However, moon-mediated visibility could also affect travel, as darkness could hamper the manoeuvres used for dynamic soaring, as suggested by Dias et al. (2012b) to explain the stronger effect of the moon on the flight activity of Cory’s shearwaters during migration than in the non-breeding residency period. Finally, it has been suggested in other organisms that the moon could help navigation [in insects, Baker (1987), Dacke et al. (2003, 2011) or crustaceans, Ugolini (2016)], although the considerable debate about the underlying mechanisms (detection of landmarks, moon compass or light-mediated magnetic compass, orientation using polarised light) (see Muheim and Jenni, 1999 for a review), and the extent to which they could apply to seabirds remains unknown. In our study, all configurations were observed (moon effect during migration only, the non-breeding period only, both, or neither; Figure 7), making a general interpretation difficult. Nevertheless, that many species showed an increase in flight bout duration during full moon (Supplementary Figure 4) suggests that bright moon illumination favours travel more than foraging.
Overall, this study provides the most comprehensive analysis to date of how seabirds adjust their activity budgets during migration, and highlights greater flexibility in their behaviour than that of terrestrial migrants. Indeed, while we found a general increase in flight activity during migration, all species took regular stops. Several species also adjusted their daily schedules to migration: diurnal species becoming more nocturnal, and vice versa for nocturnal species. Finally, moon phase affected the flight activity of many species, mostly increasing nocturnality, but the effect was similarly pronounced when birds were migrating and when in non-breeding areas. The study also raises new questions about the main drivers of the variation among species in daily activity budgets during migration, the energetic implications of these changes in time budgets, and the effects of moon phase on travel and foraging in birds.
Data Availability Statement
The data analysed in this study is subject to the following licenses/restrictions: Data requests should be sent directly to the authors. Requests to access these datasets should be directed to PC (paulo.catry@gmail.com) and will be redirected to the appropriate data holders.
Ethics Statement
Ethical review and approval was not required for the animal study because this study does not use newly collected data on vertebrate animals, but only re-uses already collected data, which received ethical approval at the time of collection.
Author Contributions
PC, MPD, RAP, and JPG conceived the ideas and contributed to the writing. All authors except A-SB-L collected the data. A-SB-L and MPD analysed the data. A-SB-L led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Funding
Funding for this work was provided by the Fundação para a Ciência e a Tecnologia (FCT, Portugal) through project Seamigrant PTDC/BIA-ANM/3743/2014 and strategic project UIDB/04292/2020 and UIDP/04292/2020 granted to MARE, and UIDP/50017/2020 and UIDB/50017/2020 granted to CESAM. Zackenberg thanks Aage V. Jensen Charity foundation; field work at Hochstetter Forland was funded by the French Polar Institute-IPEV (program “Interactions-1036”). The work on long-tailed skuas in Svalbard was funded by the Fram Center flagship “Climate Change in Fjord and Coast” grant no. 232019. The work on Kongsfjord kittiwakes was funded by the French Polar Institute-IPEV (program “Ornitho-Endocrino-330”). Fieldwork at Bird Island (South Georgia) was part of the Ecosystems component of the British Antarctic Survey Polar Science for Planet Earth Programme, funded by the Natural Environment Research Council. Funding for data collection on sooty shearwaters included an NSERC Discovery Grant and Government of Canada’s Program for International Polar Year to WM. The study on Leach’s storm-petrel was supported by Environment and Climate Change Canada. Research at Gough Island on great shearwaters was conducted with permission from the Tristan government and with support from the South African National Antarctic Programme. Funding for data collection on Cape Verde shearwaters was provided by the project Alcyon – Conservation of seabirds from Cabo Verde, coordinated by BirdLife International and funded by the MAVA Fondation pour la nature (MAVA17022), by the Programa ICREA (Acadèmia de la Institució Catalana de Recerca i Estudis Avançats), and by the projects CGL2006-01315/BOS; CGL2009-11278/BOS; CGL2013-42585-P, and CGL2016-78530-R from the Spanish Government.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Maria Alho for data cleaning. For long-tailed skua data from NE Greenland, we thank B. Sittler for organizing expeditions to Karupelv Valley and M. Nitze, F. Normann, and A. Lang for assistance in the field. For the work on kittiwakes, we thank Frédéric Angelier, Pierre Blévin, Aurélie Goutte, and Sabrina Tartu for their great help in the field. For data collection on sooty shearwaters, we thank the Falkland Islands Government for research and access permits, and Falklands Conservation, Alastair Baylis, Helen Otley, Sally Poncet, Robin Snape, and Anton Wolfaardt for field and logistic support. For data collection on Leach’s storm-petrel, we are grateful to Chantelle Burke, Laura McFarlane Tranquilla, and Cerren Richards for help in the field, Tony Doyle for transport to the island, and the Parks Division of the Government of Newfoundland and Labrador’s Fisheries and Lands Department for research and access permits for the Baccalieu Island Seabird Ecological Reserve.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.683071/full#supplementary-material
References
Adamík, P., Emmenegger, T., Briedis, M., Gustafsson, L., Henshaw, I., Krist, M., et al. (2016). Barrier crossing in small avian migrants: individual tracking reveals prolonged nocturnal flights into the day as a common migratory strategy. Sci. Rep. 6:21560. doi: 10.1038/srep21560
Alerstam, T. (2009). Flight by night or day? Optimal daily timing of bird migration. J. Theor. Biol. 258, 530–536. doi: 10.1016/j.jtbi.2009.01.020
Baker, R. R. (1987). Integrated use of moon and magnetic compasses by the heart-and-dart moth, Agrotis exclamationis. Anim. Behav. 35, 94–101. doi: 10.1016/S0003-3472(87)80214-2
Berg, M., Linnebjerg, J. F., Taylor, G., Ismar-Rebitz, S. M. H., Bell, M., Gaskin, C. P., et al. (2019). Year-round distribution, activity patterns and habitat use of a poorly studied pelagic seabird, the fluttering shearwater Puffinus gavia. PLoS One 14:e0219986. doi: 10.1371/journal.pone.0219986
Biebach, H., Biebach, I., Friedrich, W., Heine, G., Partecke, J., and Schmidl, D. (2000). Strategies of passerine migration across the Mediterranean Sea and the Sahara Desert: a radar study. IBIS 142, 623–634. doi: 10.1111/j.1474-919X.2000.tb04462.x
Bridge, E. S. (2007). Influences of morphology and behavior on wingmolt strategies in seabirds. Mar. Ornithol. 34, 7–19.
Cherel, Y., Quillfeldt, P., Delord, K., and Weimerskirch, H. (2016). Combination of At-Sea Activity, geolocation and feather stable isotopes documents where and when seabirds molt. Front. Ecol. Evol. 4:3. doi: 10.3389/fevo.2016.00003
Clarke, J. (1983). Moonlight’s influence on predator/prey interactions between short-eared owls (Asio flammeus) and deermice (Peromyscus maniculatus). Behav. Ecol. Sociobiol. 13, 205–209. doi: 10.1007/BF00299924
Clay, T., Phillips, R., Manica, A., Jackson, H., and Brooke, M. (2017). Escaping the oligotrophic gyre? The year-round movements, foraging behaviour and habitat preferences of Murphy’s petrels. Mar. Ecol. Prog. Ser. 579, 139–155. doi: 10.3354/meps12244
Covino, K. M., and Cooney, B. (2015). Daytime sleeping behavior observed in a Black-and-white Warbler during spring stopover. Anim. Migr. 2, 44–46. doi: 10.1515/ami-2015-0001
Cruz, S. M., Hooten, M., Huyvaert, K. P., Proaño, C. B., Anderson, D. J., Afanasyev, V., et al. (2013). At–sea behavior varies with lunar phase in a nocturnal pelagic seabird, the swallow-tailed gull. PLoS One 8:e56889. doi: 10.1371/journal.pone.0056889
Dacke, M., Byrne, M. J., Baird, E., Scholtz, C. H., and Warrant, E. J. (2011). How dim is dim? Precision of the celestial compass in moonlight and sunlight. Philos. Trans. R. Soc. B Biol. Sci. 366, 697–702. doi: 10.1098/rstb.2010.0191
Dacke, M., Nilsson, D.-E., Scholtz, C. H., Byrne, M., and Warrant, E. J. (2003). Insect orientation to polarized moonlight. Nature 424, 33–33. doi: 10.1038/424033a
Delord, K., Kato, A., Tarroux, A., Orgeret, F., Cotté, C., Ropert-Coudert, Y., et al. (2020). Antarctic petrels ‘on the ice rocks’: wintering strategy of an Antarctic seabird. R. Soc. Open Sci. 7:191429. doi: 10.1098/rsos.191429
Dias, M. P., Granadeiro, J. P., and Catry, P. (2012a). Do seabirds differ from other migrants in their travel arrangements? On route strategies of cory’s shearwater during its trans-equatorial journey. PLoS One 7:e49376. doi: 10.1371/journal.pone.0049376
Dias, M. P., Granadeiro, J. P., and Catry, P. (2012b). Working the day or the night shift? Foraging schedules of Cory’s shearwaters vary according to marine habitat. Mar. Ecol. Prog. Ser. 467, 245–252. doi: 10.3354/meps09966
Dias, M. P., Romero, J., Granadeiro, J. P., Catry, T., Pollet, I. L., and Catry, P. (2016). Distribution and at-sea activity of a nocturnal seabird, the Bulwer’s petrel Bulweria bulwerii, during the incubation period. Deep Sea Res. Part Oceanogr. Res. Pap. 113, 49–56. doi: 10.1016/j.dsr.2016.03.006
Egevang, C., Stenhouse, I. J., Phillips, R. A., Petersen, A., Fox, J. W., and Silk, J. R. D. (2010). Tracking of arctic terns Sterna paradisaea reveals longest animal migration. Proc. Natl. Acad. Sci.U.S.A. 107, 2078–2081. doi: 10.1073/pnas.0909493107
Evens, R., Kowalczyk, C., Norevik, G., Ulenaers, E., Davaasuren, B., Bayargur, S., et al. (2020). Lunar synchronization of daily activity patterns in a crepuscular avian insectivore. Ecol. Evol. 10, 7106–7116. doi: 10.1002/ece3.6412
Fayet, A. L., Freeman, R., Shoji, A., Boyle, D., Kirk, H. L., Dean, B. J., et al. (2016). Drivers and fitness consequences of dispersive migration in a pelagic seabird. Behav. Ecol. 27, 1061–1072. doi: 10.1093/beheco/arw013
Fayet, A. L., Shannon, P., Lyons, D. E., and Kress, S. W. (2020). Manx shearwaters Puffinus puffinus breeding in the western Atlantic follow a different migration route from their eastern Atlantic conspecifics. Mar. Ornithol. 48, 179–183.
Felicísimo, ÁM., Muñoz, J., and González-Solis, J. (2008). Ocean surface winds drive dynamics of transoceanic aerial movements. PLoS One 3:e2928. doi: 10.1371/journal.pone.0002928
Fernandez-Duque, E. (2003). Influences of moonlight, ambient temperature, and food availability on the diurnal and nocturnal activity of owl monkeys (Aotus azarai). Behav. Ecol. Sociobiol. 54, 431–440. doi: 10.1007/s00265-003-0637-9
Gill, R. E., Tibbitts, T. L., Douglas, D. C., Handel, C. M., Mulcahy, D. M., Gottschalck, J. C., et al. (2009). Extreme endurance flights by landbirds crossing the Pacific Ocean: ecological corridor rather than barrier? Proc. R. Soc. Lond. B Biol. Sci. 276, 447–457. doi: 10.1098/rspb.2008.1142
Gudmundsson, G. A., Benvenuti, S., Alerstam, T., Papi, F., Lilliendahl, K., and Åkesson, S. (1995). Examining the limits of flight and orientation performance: satellite tracking of brent geese migrating across the Greenland ice-cap. Proc. R. Soc. Lond. B Biol. Sci. 261, 73–79. doi: 10.1098/rspb.1995.0119
Guglielmo, C. G., Piersma, T., and Williams, T. D. (2001). A sport-physiological perspective on bird migration: evidence for flight-induced muscle damage. J. Exp. Biol. 204, 2683–2690.
Gutowsky, S. E., Gutowsky, L. F., Jonsen, I. D., Leonard, M. L., Naughton, M. B., Romano, M. D., et al. (2014). Daily activity budgets reveal a quasi-flightless stage during non-breeding in Hawaiian albatrosses. Mov. Ecol. 2:23. doi: 10.1186/s40462-014-0023-4
Hedd, A., Montevecchi, W., Otley, H., Phillips, R., and Fifield, D. (2012). Trans-equatorial migration and habitat use by sooty shearwaters Puffinus griseus from the South Atlantic during the nonbreeding season. Mar. Ecol. Prog. Ser. 449, 277–290. doi: 10.3354/meps09538
Hill, H. D. (1994). “Theory of geolocation by light levels,” in Elephant Seals: Population Biology, Behavior, and Physiology, eds B. J. Le Boeuf and R. M. Laws (Berkeley, CA: University of California Press), 227–236.
Jackson, S. (1988). Diets of the white-chinned petrel and sooty shearwater in the Southern Benguela Region, South Africa. Condor 90, 20–28. doi: 10.2307/1368428
James, D., Jarry, G., and Érard, C. (2000). Effet de la lune sur la migration postnuptiale nocturne de l’alouette des champs Alauda arvensis L. en France. C. R. Acad. Sci. III 323, 215–224. doi: 10.1016/S0764-4469(00)00121-9
Jenni-Eiermann, S., Jenni, L., Smith, S., and Costantini, D. (2014). Oxidative stress in endurance flight: an unconsidered factor in bird migration. PLoS One 9:e97650. doi: 10.1371/journal.pone.0097650
Kelley, D., and Richards, C. (2021). oce: Analysis of Oceanographic Data. R Package Version 1.4-0. Availabe online at: https://CRAN.R-project.org/package=oce
Kerfoot, W. B. (1967). The lunar periodicity of Sphecodogastra texana, a nocturnal bee (Hymenoptera: Halictidae). Anim. Behav. 15, 479–486. doi: 10.1016/0003-3472(67)90047-4
Klaassen, R. H. G., Strandberg, R., Hake, M., and Alerstam, T. (2008). Flexibility in daily travel routines causes regional variation in bird migration speed. Behav. Ecol. Sociobiol. 62, 1427–1432. doi: 10.1007/s00265-008-0572-x
Krapu, G. L., Eldridge, J. L., Gratto-Trevor, C. L., and Buhl, D. A. (2006). Fat dynamics of arctic-nesting sandpipers during spring in mid-continental North America (Dinámica de la Grasa en Chorlos que Nidifican en el Ártico durante la Primavera en el Área Continental Central de América del Norte). Auk 123, 323–334.
Lamb, H. H. (1975). Our understanding of the global wind circulation and climatic variations. Bird Study 22, 121–141. doi: 10.1080/00063657509476457
Landers, T. J., Rayner, M. J., Phillips, R. A., and Hauber, M. E. (2011). Dynamics of seasonal movements by a trans-Pacific Migrant, the Westland Petrel. Condor 113, 71–79. doi: 10.1525/cond.2011.100064
Lemke, H. W., Tarka, M., Klaassen, R. H. G., Ãkesson, M., Bensch, S., Hasselquist, D., et al. (2013). Annual cycle and migration strategies of a trans-saharan migratory songbird: a geolocator study in the great reed warbler. PLoS One 8:e79209. doi: 10.1371/journal.pone.0079209
Mackley, E., Phillips, R., Silk, J., Wakefield, E., Afanasyev, V., Fox, J., et al. (2010). Free as a bird? Activity patterns of albatrosses during the nonbreeding period. Mar. Ecol. Prog. Ser. 406, 291–303. doi: 10.3354/meps08532
Mackley, E. K., Phillips, R. A., Silk, J. R. D., Wakefield, E. D., Afanasyev, V., and Furness, R. W. (2011). At-sea activity patterns of breeding and nonbreeding white-chinned petrels Procellaria aequinoctialis from South Georgia. Mar. Biol. 158, 429–438. doi: 10.1007/s00227-010-1570-x
Mann, K. H., and Lazier, J. R. N. (2005). Dynamics of Marine Ecosystems: Biological–Physical Interactions in the Oceans. Malden, MA: Wiley-Blackwell.
Muheim, R., and Jenni, L. (1999). Nocturnal orientation of robins, Erithacus rubecula: birds caught during migratory flight are disoriented. Acta Ethologica 2, 43–50. doi: 10.1007/PL00012231
Navarro, J., Votier, S. C., Aguzzi, J., Chiesa, J. J., Forero, M. G., and Phillips, R. A. (2013). Ecological segregation in space, time and trophic niche of sympatric planktivorous petrels. PLoS One 8:e62897. doi: 10.1371/journal.pone.0062897
Németh, Z. (2009). Observation of daytime sleep-like behavior in a migratory songbird during stopover. Wilson J. Ornithol. 121, 644–646.
Norevik, G., Åkesson, S., Andersson, A., Bäckman, J., and Hedenström, A. (2019). The lunar cycle drives migration of a nocturnal bird. PLoS Biol. 17:e3000456. doi: 10.1371/journal.pbio.3000456
Pastor-Prieto, M., Ramos, R., Zajková, Z., Reyes-González, J., Rivas, M., Ryan, P., et al. (2019). Spatial ecology, phenological variability and moulting patterns of the Endangered Atlantic petrel Pterodroma incerta. Endanger. Species Res. 40, 189–206. doi: 10.3354/esr00991
Penteriani, V., Kuparinen, A., Delgado, M., Palomares, F., López-Bao, J. V., Fedriani, J., et al. (2013). Responses of a top and a meso predator and their prey to moon phases. Oecologia 173, 753–766. doi: 10.1007/s00442-013-2651-6
Phalan, B., Phillips, R. A., Silk, J. R., Afanasyev, V., Fukuda, A., Fox, J., et al. (2007). Foraging behaviour of four albatross species by night and day. Mar. Ecol. Prog. Ser. 340, 271–286.
Phillips, R., Lewis, S., González-Solís, J., and Daunt, F. (2017). Causes and consequences of individual variability and specialization in foraging and migration strategies of seabirds. Mar. Ecol. Prog. Ser. 578, 117–150. doi: 10.3354/meps12217
Phillips, R. A., Silk, J. R. D., Croxall, J. P., Afanasyev, V., and Briggs, D. R. (2004). Accuracy of geolocation estimates for flying seabirds. Mar. Ecol. Prog. Ser. 266, 265–272. doi: 10.3354/meps266265
Piersma, T., Gudmundsson, G. A., and Lilliendahl, K. (1999). Rapid changes in the size of different functional organ and muscle groups during refueling in a long-distance migrating shorebird. Physiol. Biochem. Zool. PBZ 72, 405–415. doi: 10.1086/316680
Pinet, P., Jaeger, A., Cordier, E., Potin, G., and Corre, M. L. (2011). Celestial moderation of tropical seabird behavior. PLoS One 6:e27663. doi: 10.1371/journal.pone.0027663
Pinheiro, J., Bates, D., DebRoy, S., and Sarkar, D., R Core Team. (2021). nlme: Linear and Nonlinear Mixed Effects Models. Available online at: < https://CRAN.R-project.org/package=nlme> (accessed Septemper 07, 2021).
Pyle, P., Nur, N., Henderson, R. P., and DeSante, D. F. (1993). The effects of weather and lunar cycle on nocturnal migration of landbirds at Southeast Farallon Island. Calif. Condor 95, 343–361. doi: 10.2307/1369357
R Core Team (2019). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Ramírez, I., Paiva, V. M., Phillips, R., Ramos, J., and Garthe, S. (2013). Year-round distribution and habitat preferences of the Bugio Petrel. Mar. Ecol. Prog. Ser. 476, 269–284. doi: 10.3354/meps10083
Ramos, R., Militão, T., González-Solís, J., and Ruiz, X. (2009). Moulting strategies of a long-distance migratory seabird, the Mediterranean Cory’s Shearwater Calonectris diomedea diomedea. IBIS 151, 151–159. doi: 10.1111/j.1474-919X.2008.00877.x
Rattenborg, N. C., Mandt, B. H., Obermeyer, W. H., Winsauer, P. J., Huber, R., Wikelski, M., et al. (2004). Migratory sleeplessness in the white-crowned sparrow (Zonotrichia leucophrys gambelii). PLoS Biol. 2:e212. doi: 10.1371/journal.pbio.0020212
Rattenborg, N. C., Voirin, B., Cruz, S. M., Tisdale, R., Dell’Omo, G., Lipp, H.-P., et al. (2016). Evidence that birds sleep in mid-flight. Nat. Commun. 7:12468. doi: 10.1038/ncomms12468
Ravache, A., Bourgeois, K., Thibault, M., Dromzée, S., Weimerskirch, H., de Grissac, S., et al. (2020). Flying to the moon: lunar cycle influences trip duration and nocturnal foraging behavior of the wedge-tailed shearwater Ardenna pacifica. J. Exp. Mar. Biol. Ecol. 525, 151322. doi: 10.1016/j.jembe.2020.151322
Rayner, M. J., Carlile, N., Priddel, D., Bretagnolle, V., Miller, M. G. R., Phillips, R. A., et al. (2016). Niche partitioning by three Pterodroma petrel species during non-breeding in the equatorial Pacific Ocean. Mar. Ecol. Prog. Ser. 549, 217–229. doi: 10.3354/meps11707
Regular, P. M., Hedd, A., and Montevecchi, W. A. (2011). Fishing in the dark: a pursuit-diving seabird modifies foraging behaviour in response to nocturnal light levels. PLoS One 6:e26763. doi: 10.1371/journal.pone.0026763
Ropert-Coudert, Y., Grémillet, D., Kato, A., Ryan, P. G., Naito, Y., and Le Maho, Y. (2004). A fine-scale time budget of Cape gannets provides insights into the foraging strategies of coastal seabirds. Anim. Behav. 67, 985–992. doi: 10.1016/j.anbehav.2003.09.010
Rubolini, D., Maggini, I., Ambrosini, R., Imperio, S., Paiva, V. H., Gaibani, G., et al. (2015). The effect of moonlight on Scopoli’s shearwater Calonectris diomedea colony attendance patterns and nocturnal foraging: a test of the foraging efficiency hypothesis. Ethology 121, 284–299. doi: 10.1111/eth.12338
Sakamoto, K. Q., Takahashi, A., Iwata, T., Yamamoto, T., Yamamoto, M., and Trathan, P. N. (2013). Heart rate and estimated energy expenditure of flapping and gliding in black-browed albatrosses. J. Exp. Biol. 216, 3175–3182. doi: 10.1242/jeb.079905
Schaub, M., and Jenni, L. (2000). Body mass of six long-distance migrant passerine species along the autumn migration route. J. Für Ornithol. 141, 441–460. doi: 10.1046/j.1439-0361.2000.00037.x
Schwilch, R., Piersma, T., Holmgren, N. M. A., and Jenni, L. (2002). Do migratory birds need a nap after a long non-stop flight? Ardea 90, 149–154.
Speicher, J., Schreffler, L., and Speicher, D. (2011). Lunar Influence on the Fall Migration of Northern Saw-whet Owls. Wilson J. Ornithol. 123, 158–160. doi: 10.1676/09-108.1
Strandberg, R., and Alerstam, T. (2007). The strategy of fly-and-forage migration, illustrated for the Osprey (Pandion haliaetus). Behav. Ecol. Sociobiol. 61, 1865–1875. doi: 10.1007/s00265-007-0426-y
Strandberg, R., Klaassen, R. H. G., Olofsson, P., and Alerstam, T. (2009). Daily travel schedules of adult eurasian hobbies falco subbuteo — variability in flight hours and migration speed along the route. Ardea 97, 287–295. doi: 10.5253/078.097.0304
Tucker, V. A. (1991). The Effect of Molting on the Gliding Performance of a Harris’, hawk (Parabuteo unicinctus). Auk 108, 108–113. doi: 10.1093/auk/108.1.108
Ugolini, A. (2016). The moon orientation of the equatorial sandhopper Talorchestia martensii Weber. Behav. Ecol. Sociobiol. 70, 1699–1706. doi: 10.1007/s00265-016-2175-2
van Bemmelen, R. S. A., Kolbeinsson, Y., Ramos, R., Gilg, O., Alves, J. A., Smith, M., et al. (2019). A migratory divide among red-necked phalaropes in the western palearctic reveals contrasting migration and wintering movement strategies. Front. Ecol. Evol. 7:86. doi: 10.3389/fevo.2019.00086
Weimerskirch, H. (2007). Are seabirds foraging for unpredictable resources? Deep Sea Res. Part II Top. Stud. Oceanogr. 54, 211–223. doi: 10.1016/j.dsr2.2006.11.013
Weimerskirch, H., Bishop, C., Jeanniard-du-Dot, T., Prudor, A., and Sachs, G. (2017). Frigate birds track atmospheric conditions over months-long transoceanic flights and perform flights inside clouds. Science 41:8.
Weimerskirch, H., de Grissac, S., Ravache, A., Prudor, A., Corbeau, A., Congdon, B., et al. (2020). At-sea movements of wedge-tailed shearwaters during and outside the breeding season from four colonies in New Caledonia. Mar. Ecol. Prog. Ser. 633, 225–238. doi: 10.3354/meps13171
Weimerskirch, H., Guionnet, T., Martin, J., Shaffer, S. A., and Costa, D. P. (2000). Fast and fuel efficient? Optimal use of wind by flying albatrosses. Proc. R. Soc. B Biol. Sci. 267, 1869–1874. doi: 10.1098/rspb.2000.1223
Yamamoto, T., Takahashi, A., Katsumata, N., Sato, K., and Trathan, P. N. (2010). At-Sea Distribution and Behavior of Streaked Shearwaters (Calonectris leucomelas) During the Nonbreeding Period. Auk 127, 871–881. doi: 10.1525/auk.2010.10029
Yamamoto, T., Takahashi, A., Yoda, K., Katsumata, N., Watanabe, S., Sato, K., et al. (2008). The lunar cycle affects at-sea behaviour in a pelagic seabird, the streaked shearwater, Calonectris leucomelas. Anim. Behav. 76, 1647–1652. doi: 10.1016/j.anbehav.2008.07.019
Keywords: bird migration, ecological barriers, nocturnality, migratory behaviour, moon phases, transequatorial migrants
Citation: Bonnet-Lebrun A-S, Dias MP, Phillips RA, Granadeiro JP, Brooke MdL, Chastel O, Clay TA, Fayet AL, Gilg O, González-Solís J, Guilford T, Hanssen SA, Hedd A, Jaeger A, Krietsch J, Lang J, Le Corre M, Militão T, Moe B, Montevecchi WA, Peter H-U, Pinet P, Rayner MJ, Reid T, Reyes-González JM, Ryan PG, Sagar PM, Schmidt NM, Thompson DR, van Bemmelen R, Watanuki Y, Weimerskirch H, Yamamoto T and Catry P (2021) Seabird Migration Strategies: Flight Budgets, Diel Activity Patterns, and Lunar Influence. Front. Mar. Sci. 8:683071. doi: 10.3389/fmars.2021.683071
Received: 19 March 2021; Accepted: 29 September 2021;
Published: 29 October 2021.
Edited by:
Adrian C. Gleiss, Murdoch University, AustraliaReviewed by:
Karissa Opal Lear, Murdoch University, AustraliaNatalie Elizabeth Wildermann, Texas A&M University–Corpus Christi, United States
Copyright © 2021 Bonnet-Lebrun, Dias, Phillips, Granadeiro, Brooke, Chastel, Clay, Fayet, Gilg, González-Solís, Guilford, Hanssen, Hedd, Jaeger, Krietsch, Lang, Le Corre, Militão, Moe, Montevecchi, Peter, Pinet, Rayner, Reid, Reyes-González, Ryan, Sagar, Schmidt, Thompson, van Bemmelen, Watanuki, Weimerskirch, Yamamoto and Catry. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anne-Sophie Bonnet-Lebrun, anne-sophie.bonnet-lebrun@normale.fr
 Anne-Sophie Bonnet-Lebrun
Anne-Sophie Bonnet-Lebrun Maria P. Dias
Maria P. Dias Richard A. Phillips
Richard A. Phillips José P. Granadeiro
José P. Granadeiro M. de L. Brooke5
M. de L. Brooke5  Annette L. Fayet
Annette L. Fayet Olivier Gilg
Olivier Gilg Sveinn A. Hanssen
Sveinn A. Hanssen Audrey Jaeger
Audrey Jaeger Teresa Militão
Teresa Militão Børge Moe
Børge Moe William A. Montevecchi
William A. Montevecchi Patrick Pinet
Patrick Pinet José Manuel Reyes-González
José Manuel Reyes-González Peter G. Ryan
Peter G. Ryan Niels M. Schmidt
Niels M. Schmidt Rob van Bemmelen
Rob van Bemmelen Yutaka Watanuki
Yutaka Watanuki Henri Weimerskirch
Henri Weimerskirch Takashi Yamamoto
Takashi Yamamoto Paulo Catry
Paulo Catry