Screening Tools for Sarcopenia in Mild to Moderate Parkinson’s Disease: Assessing the Accuracy of SARC-F and Calf Circumference
Abstract
Background:
Parkinson’s disease (PD) and sarcopenia share similar pathophysiological mechanisms.
Objective:
Estimate the prevalence of sarcopenia in PD patients and describe clinical and demographic features associated with sarcopenia.
Methods:
A cross-sectional study was carried out at a tertiary public hospital in Brazil. A modified HY scale of stage 1 to 3, being at least 40 years old and having the ability to stand and walk unassisted were required for eligibility. We evaluated physical performance and muscle mass using DEXA.
Results:
The study population comprised 124 patients, of which 53 (42.7%) were women. The mean age and mean disease duration were 65.8±10.5 and 10.1±5.8 years, respectively. The mean handgrip strength of 20.4±6.9 in woman and 34.6±8.4 kg in men. Moreover, 50.8% patients had positive SARC-F, 20% patients had probable sarcopenia, 9.6% confirmed sarcopenia, and 16.8% patients showed low muscle mass quantity measured by DEXA. Lower Levodopa Equivalent Dosage (LED) and calf circumference (CC) were independently associated with confirmed sarcopenia. LLED, higher MDS-UPDRS Part III, and lower MMSE scores were independently associated with probable sarcopenia. The CC demonstrated accuracy to identify PD patients with confirmed sarcopenia with a cut-off of <31 cm in women and <34 cm in men.
Conclusion:
We found low prevalence of confirmed sarcopenia among PD patients. We propose that healthcare providers introduce measuring CC, which is a quick and inexpensive method to assess for sarcopenia in PD patients.
INTRODUCTION
Sarcopenia is a generalized muscle disorder currently defined by the occurrence of low muscle strength as well as low muscle quantity or quality. It has a well-established association with several adverse outcomes such as falls, fractures, disability, poor quality of life, institutionalization, hospitalization, and death [1, 2].
Parkinson’s disease (PD) is a chronic and progressive neurodegenerative disease whose prevalence increases with aging. It usually progresses over time and is related to a decline in physical function and loss of functionality as a consequence of several motor and non-motor symptoms [3].
Kwan [4] suggested that the pathophysiology of sarcopenia can be neurogenic, musculogenic (a term coined to distinguish it from “myogenic”), synaptogenic (from the neuromuscular junctions), or vasculogenic (from blood vessels). Firstly, age-related declines in motor axon conduction velocity and myelinated axon density have been observed. Aging is associated with a decrease in motor unit reinnervation after denervation, as well as other factors [4]. In addition, PD and sarcopenia share similar pathophysiological mechanisms, including, but not limited to inflammation, muscle autophagy, oxidative stress, and apoptosis, which may all cause muscle fiber loss [5]. Few studies have assessed the occurrence of sarcopenia in PD [6]. Furthermore, recent changes in sarcopenia definitions and diagnostic criteria induce significant heterogeneity of findings in different studies [5, 6].
In view of the above, the aim of the present study was to estimate the prevalence of probable sarcopenia, confirmed sarcopenia and severe sarcopenia in PD patients according to European Working Group on Sarcopenia in Older People 2 (EWGSOP 2) diagnostic criteria and to describe the clinical and demographic features associated with sarcopenia. In addition, we also evaluated the SARC-F questionnaire and right calf circumference to screen for sarcopenia in patients with PD.
METHODS
Study participants
A cross-sectional study was carried out at Hospital Universitario Walter Cantidio (HUWC) in Fortaleza, Brazil, from May 2021 to April 2022. The sample was composed of patients with PD who regularly attended the Movement Disorders outpatient clinic of the HUWC.
PD was diagnosed according to the Movement Disorders Society (MDS) criteria [7] by two neurologists and one geriatrician specialized in PD. A Parkinson’s disease clinical diagnosis and a disease severity score of stage 1 to 3 on the modified Hoehn and Yahr (HY) scale were required for eligibility, being at least 40 years of age and having the ability to stand and walk unassisted. We did not include patients with severe disease (HY 4 and 5) because they would be unable to complete the Five Times Sit-to-Stand (FTSTS), balance tests, or gait speed tests. Patients with HY 5 are no longer seen in the outpatient clinic for face-to-face consultations. Home care is recommended for them. Patients with severe health conditions or uncontrolled chronic diseases that could compromise their safety or impact how the data were interpreted were excluded as follows:
- Heart failure with the New York Heart Association (NYHA) Functional Classification class III (less than ordinary activity causes fatigue, palpitation, or dyspnea) and IV (symptoms of heart failure at rest);
- Dialysis-dependent end-stage renal disease;
- Neurological diseases (non-PD) with motor impairment;
- Severe (dyspnea at mild exertion) or very severe (dyspnea at rest and/or oxygen therapy) chronic obstructive pulmonary disease;
- Severe knee, wrist, hand, or spine osteoarthritis;
- Cancer diagnosis, except localized prostate cancer and localized skin cancer;
- Moderate to severe dementia (CDR 2 and 3).
We also excluded conditions that would hamper the interpretation of the Dual Energy X-ray Absorptiometry (DEXA):
- Recently administered gastrointestinal contrast or radionuclides (last 72 hours);
- Pregnancy;
- Deep Brain Stimulation;
- Heart pacemaker.
All participants provided written informed consent for the study, which was authorized by the Hospital Universitário Walter Cantidio Research Ethics Committee (register number 91075318.1.0000.5045). The study’s researchers spoke with and assessed each patient.
Clinical assessment
We employed an interview that was structured to gather sociodemographic and medical data. We assessed prior histories of hypertension, diabetes, and depression according to the Diagnostic and Statistical Manual of Mental Disorders, V (DSM-V), dementia (DSM-V), and osteoporosis according to the recommendations from the National Osteoporosis Foundation. The clinical data obtained from the patients were compared with information from their relatives, caregivers, and healthcare records to ensure its accuracy. We also gathered data on the antiparkinsonian medications provided by the Brazilian public health system used such as L-dopa, COMT inhibitors (entacapone), MAO-B inhibitors (rasagiline), amantadine, and dopamine agonists (pramipexole), as well as L-dopa formulations such as L-dopa/carbidopa, L-dopa/benserazide, and controlled-release L-dopa formulations. We defined the levodopa equivalent dose (LED) of an antiparkinsonian drug as the dose which produces the same level of symptomatic control as 100 mg of immediate release L-dopa according to the systematic review conducted by Tomlinson et al. [8]. We used the Schwab and England Activities of Daily Living (SE ADL) Scale to evaluate ADL, the modified Hoehn and Yahr (HY) staging to assess PD severity, and the Movement Disorders Society-Unified Parkinson’s Disease Rating Scale part III (MDS-UPDRS III) to assess severity of motor parkinsonian symptoms [9]. Depressive symptoms were assessed using the 15-item Geriatric Depression Scale (GDS-15) and cognition was evaluated through the Mini-Mental Status Examination (MMSE) [10]. A fall was described as a situation in which the patient unintentionally fell to the ground or to another lower level, and it was not due to a seizure, a vehicle or bike accident, or a syncope. Patients were questioned prior to their medical visit about any similar incidents that occurred in the preceding one and six months. Data on falls collected from patients were compared with information from relatives, caretakers, and clinical records for accuracy purposes.
All participants were weighed without shoes on or heavy accessories such as mobile phones and wallets. The body mass index was determined by dividing the total body weight (in kilograms) by the square of the height (meters).
Sarcopenia assessment
The calf is the best site for anthropometric measurements to evaluate loss of skeletal muscle mass (SMM) and has been used to forecast SMM in some studies [11, 12]. Adult lower limbs include about 30% of their skeletal muscle. The extremities have less fat mass compared to other body regions, which lessens its influence on these parameters. The calf circumference (CC) measurement also has the advantages of being feasible, simple to carry out, and not requiring undressing [13]. The right CC was measured at the right calf’s greatest girth using an inelastic but flexible plastic tape measure with the person in a sitting position with the knee and ankle at a right angle and feet resting on the floor. The patient should remove his/her clothing on the lower body to measure the CC on bare skin. Subcutaneous tissues were not compressed [14]. We chose the right side because Jeong et al. (2020) recently showed that CC measured on the right side was greater than on the left side, regardless of the dominant hand in a sample of community-dwelling ambulatory older adults [15].
Probable sarcopenia was defined as low handgrip strength. Confirmed sarcopenia was diagnosed according to the EWGSOP 2 as follows: low muscle strength and low muscle quantity or quality, being considered severe when low muscle strength, low muscle quantity or quality, and low physical performance are evidenced [1]. The presence of probable sarcopenia is required in the definition of confirmed sarcopenia. Confirmed sarcopenia is defined as a loss of both muscle mass and strength. Probable sarcopenia is defined as a loss of strength, and it is the main and most definitive measure of muscular function. Probable sarcopenia may be utilized in clinical practice to guide in the diagnosis of definite sarcopenia and in the implementation of early intervention. A muscular strength test, such as handgrip strength, is advised as the first step in diagnosing sarcopenia and identifying persons at risk. A further finding of poor muscle mass or quality confirms the diagnosis of sarcopenia. However, low strength is deemed sufficient to commence actions [16].
The SARC-F was administered to all patients. The SARC-F is a simple and affordable method for sarcopenia risk assessment that is easily applied in community healthcare settings and other clinical settings. It contains 5 items to gauge how the patient feels about their physical limits, including their ability to walk, carry a 5-pound object, stand up from a chair, climb stairs, and falling [17].
We followed the recommendations of the EWGSOP2 regarding the handgrip strength measure and cut-offs (<27 kg for men and <16 kg for women). We used the SAEHAN® dynamometer following the Southhampton protocol (patient seated with their forearms resting on the arms of the chair, wrist just over the end of the chair arm, in a neutral position, thumb facing upwards, feet flat on the floor, three trials on each side, alternating sides, maximal grip score from all six trials used) [18]. Bradykinesia, stiffness, and tremor may be present unilaterally or bilaterally in PD due to PD asymmetries, therefore the stronger handgrip strength was taken into account, as done by Vetrano et al. [5].
The Short Physical Performance Battery test was used to evaluate physical performance [19]. Measures of standing balance, 4-m gait speed, and the amount of time required to get up from a chair five times are all part of the test. Patients were instructed to maintain their balance by standing with their feet together before spending 10 seconds in each of the semi-tandem and tandem positions, which include placing one foot’s heel next to the other foot’s big toe. Participants were instructed to walk along an 8-m track (consisting of 2-m of acceleration and 2-m of deceleration) at their normal pace to measure their gait speed using a stopwatch. Participants were instructed to stand up and sit down five times as rapidly as they could with their arms crossed over their chests to test their ability to get out of a chair. This was not done until after individuals had shown they could stand up once without using their arms. The overall SPPB score was calculated [20], for which a score of less than 8 points indicates inadequate physical performance, and the highest score is 12.
Muscle mass was determined using DEXA to estimate appendicular skeletal muscle mass (ASMM) adjusted for height in meters squared to obtain the lean mass index (LMI = ASMM/Ht2). Lean mass measures in the arms and legs were used to quantify appendicular lean mass. Appendicular lean mass measurements for people whose body parts were unilaterally harmed were obtained by twice the values for the unaffected side [21]. Low muscle mass was defined as ASMM index <7 kg/m2 for men and <5.5 kg/m2 for women according to EWGSOP 2. All patients were evaluated for disease staging, UPDRS III, GDS-15, MMSE, SPPB, and handgrip strength during “on” phases.
Statistical analysis
Descriptive statistics were presented as numbers (percentage) for categorical variables and as mean±standard deviation (median) for quantitative variables. Bivariate analysis for probable and confirmed sarcopenia were performed using the Pearson’s chi-squared test and Fisher’s exact test for categorial variables. The Mann-Whitney U test was used to assess quantitative independent variables since they were not normally distributed except for CC, for which Student’s T-test was used. Variables with p < 0.05 entered forward stepwise logistic regression to identify those independently associated with probable and confirmed sarcopenia. The sensitivity and specificity of SARC-F and CC for identifying confirmed sarcopenia were calculated. In addition, we used receiver operating characteristics (ROC) curves to obtain cut-off values and calculated the area under the curve (AUC) and 95% confidence interval (CI). Sensitivity, specificity, positive (PPVs) and negative predictive values (NPVs) were calculated for different cut-off scores of CC to identify sarcopenia in men and women. Statistical analyses were performed using SPSS v 21.0 program (SPSS Statistics; IBM, Armonk, NY).
RESULTS
Demographic characteristics and clinical background
The study population comprised a total of 124 patients, of which 53 (42.7%) were women. The mean age was 65.8±10.5 years and mean disease duration was 10.1±5.8 years. A total of 63 (50.8%) patients had positive screening for sarcopenia through the SARC-F. The sample showed mean handgrip strength of 20.4±6.9 kg in woman and of 34.6±8.4 kg in men. Moreover, 25 (20%) patients had probable sarcopenia according to low handgrip strength, while 21 (16.8%) patients showed low muscle mass quantity measured by DEXA. Next, 12 (9.6%) patients had both low handgrip strength and low muscle mass quantity, thus meeting criteria for confirmed sarcopenia. Among them, four patients also had inadequate physical performance (SPPB score ≤8 or gait speed ≤0.8 m/s). Therefore, the prevalence of severe sarcopenia in our sample was 3.22%.
Bivariate and logistic regression analyses for sarcopenia
Tables 1 and 2 show the main demographic and clinical features of the study participants, as well as the bivariate analysis for probable sarcopenia and confirmed sarcopenia. Both probable and confirmed sarcopenia were associated with older age, lower LED, and decreased CC. Probable sarcopenia was associated with higher MDS-UPDRS Part III score, lack of regular physical activity, lower SE ADL score and worse performance on the MMSE, gait speed assessment, and SPPB. Confirmed sarcopenia was associated with lower body mass index. These variables were included in the respective logistic regression models in a stepwise forward manner to identify factors independently and significantly associated with sarcopenia (Table 3). Higher levodopa equivalent dose was independently associated as a protective factor for both probable and confirmed sarcopenia (Table 3). Better cognitive performance as assessed through MMSE and low MDS-UPDRS Part III score were independently associated as protective factors for probable sarcopenia (Table 3), whereas CC was independently associated with confirmed sarcopenia (Table 3).
Table 1
Sociodemographic and clinical characteristics and bivariate analysis for probable sarcopenia
| Total | Yes | No | p | |
| Gender | ||||
| Male | 71 (57.3%) | 13 (52%) | 58 (58.6%) | 0.552a |
| Female | 53 (42.7%) | 12 (48%) | 41 (41.4%) | |
| Age | 65.8±10.5 (65.9) | 71.2±9.7 (73.5) | 64.4±10.3 (64.2) | 0.004b |
| Disease duration | 10.1±5.8 (9) | 9.6±6.2 (10) | 10.2±5.8 (9) | 0.694b |
| Hoehn and Yahr stage | 2.5±0.4 (2.5) | 2.7±0.3 (3) | 2.5±0.4 (2.5) | 0.052b |
| Family history of PD (n = 121) | 51 (42.1%) | 9 (36%) | 42 (43.8%) | 0.485a |
| MDS-UPDRS Part III score | 43.9±15.2 (44) | 50.6±13.2 (52) | 42.2±15.2 (39) | 0.015b |
| Hypertension | 59 (47.6%) | 12 (48%) | 47 (47.5%) | 0.963a |
| Type 2 DM | 14 (11.3%) | 2 (8%) | 12 (12.1%) | 0.733d |
| LED | 1138.6±573.3 (1100) | 909.2±503.7 (875) | 1196.5±577.6 (1200) | 0.007b |
| Number of medications | 5.4±2.2 (5) | 4.8±1.7 (5) | 5.5±2.3 (5) | 0.314b |
| Visual hallucinations | 26 (21%) | 6 (24%) | 20 (20.2%) | 0.677a |
| Dyskinesia | 66 (53.2%) | 11 (44%) | 55 (55.6%) | 0.301a |
| Motor fluctuations | 80 (64.5%) | 14 (56%) | 66 (66.7%) | 0.319a |
| Freezing of gait | 53 (42.7%) | 13 (52%) | 40 (40.4%) | 0.295a |
| Sleep complaints | 105 (84.7%) | 24 (96%) | 81 (81.8%) | 0.118d |
| REM Sleep Behavior Disorder | 69 (55.6%) | 13 (52%) | 56 (56.6%) | 0.681a |
| Urinary incontinence | 56 (45.2%) | 11 (44%) | 45 (45.5%) | 0.896a |
| Cognitive complaints (N = 122) | 72 (59%) | 17 (68%) | 55 (56.7%) | 0.306a |
| Alcohol use | 15 (12.1%) | 4 (16%) | 11 (11.1%) | 0.501d |
| Current cigarette smoking | 3 (2.4%) | 1 (4%) | 2 (2%) | 0.494d |
| Use of walking aid devices | 26 (21%) | 5 (20%) | 21 (21.2%) | 0.894a |
| Regular physical activity | 40 (32.3%) | 3 (12%) | 37 (37.4%) | 0.017d |
| SE ADL | 83.9±11.4 (90) | 78±16.1 (80) | 85.4±9.5 (90) | 0.029b |
| MMSE score (n = 123) | 24.1±4.3 (25) | 21.1±5.8 (22) | 24.8±3.5 (26) | 0.002b |
| GDS score (n = 123) | 5.0±3.3 (4) | 6±3.5 (6) | 4.7±3.3 (4) | 0.098b |
| Body mass index | 26.9±4.9 (26.8) | 25.4±4.4 (25.1) | 27.2±5 (26.9) | 0.086b |
| Right calf circumference (n = 121) | 33.7±3.6 (34) | 32.4±3.4 (32.5) | 34±3.6 (34.2) | 0.039c |
| SARC-F ≥4 | 63 (50.8%) | 15 (60%) | 48 (48.5%) | 0.303a |
| Gait speed (n = 122) | 1.4±0.5 (1.4) | 1.1±0.6 (0.9) | 1.4±0.5 (1.4) | 0.002b |
| SPPB score | 8.8±2.6 (9) | 7.4±2.5 (8) | 9.1±2.6 (9) | 0.003b |
| Falls in the last month | 23 (18.5%) | 4 (16%) | 19 (19.2%) | 1.000d |
| Falls in the last 6 months | 51 (41.1%) | 12 (48%) | 39 (39.4%) | 0.435a |
Data expressed as percentage (%), as well as mean±standard deviation (median); aPearsons chi-squared test; bMann-Whitney test; cStudents T test; dFishers exact test; SE ADL, Schwab and England Activities of Daily Living Scale; LED, Levodopa Equivalent Dose; GDS, Geriatric Depression Scale; MMSE, Mini-Mental State Examination; GDS, Geriatric Depression Scale; SPPB, Short Physical Performance Battery.
Table 2
Bivariate analysis for confirmed sarcopenia
| Confirmed sarcopenia | ||||
| Total | Yes | No | p | |
| Gender | ||||
| Male | 71 (57.3%) | 7 (58.3%) | 64 (57.1%) | 0.937a |
| Female | 53 (42.7%) | 5 (41.7%) | 48 (42.9%) | |
| Age | 65.8±10.5 (65.9) | 72.9±8.8 (75.4) | 65±10.4 (65.3) | 0.012b |
| Disease duration | 10.1±5.8 (9) | 9±7.6 (7) | 10.2±5.6 (9) | 0.313b |
| Hoehn and Yahr stage | 2.5±0.4 (2.5) | 2.6±0.4 (2.5) | 2.5±0.4 (2.5) | 0.596b |
| Family history of PD (n = 121) | 51 (42.1%) | 3 (25%) | 48 (44%) | 0.236d |
| MDS-UPDRS Part III score | 43.9±15.2 (44) | 48.3±12.1 (46.5) | 43.4±15.4 (42.5) | 0.261b |
| Hypertension | 59 (47.6%) | 5 (41.7%) | 54 (48.2%) | 0.666a |
| Type 2 DM | 14 (11.3%) | 1 (8.3%) | 13 (11.6%) | 1.000d |
| LED | 1138.6±573.3 (1100) | 722.9±391.5 (652) | 1183.1±573.1 (1200) | 0.008b |
| Number of medications | 5.4±2.2 (5) | 5±1.8 (5) | 5.4±2.2 (5) | 0.781b |
| Visual hallucinations | 26 (21%) | 3 (25%) | 23 (20.5%) | 0.714d |
| Dyskinesia | 66 (53.2%) | 5 (41.7%) | 61 (54.5%) | 0.398a |
| Motor fluctuations | 80 (64.5%) | 6 (50%) | 74 (66.1%) | 0.269a |
| Freezing of gait | 53 (42.7%) | 7 (58.3%) | 46 (41.1%) | 0.251a |
| Sleep complaints | 105 (84.7%) | 11 (91.7%) | 94 (83.9%) | 0.690d |
| REM Sleep Behavior Disorder | 69 (55.6%) | 9 (75%) | 60 (53.6%) | 0.224d |
| Urinary incontinence | 56 (45.2%) | 3 (25%) | 53 (47.3%) | 0.222d |
| Cognitive complaints (N = 122) | 72 (59%) | 9 (75%) | 63 (57.3%) | 0.356d |
| Alcohol use | 15 (12.1%) | 2 (16.7%) | 13 (11.6%) | 0.639d |
| Current cigarette smoking | 3 (2.4%) | 1 (8.3%) | 2 (1.8%) | 0.265d |
| Use of walking aid devices | 26 (21%) | 2 (16.7%) | 24 (21.4%) | 1.000d |
| Regular physical activity | 40 (32.3%) | 3 (25%) | 37 (33%) | 0.750d |
| SE ADL | 83.9±11.4 (90) | 81.7±15.8 (90) | 84.2±10.9 (90) | 0.837b |
| MMSE score (n = 123) | 24.1±4.3 (25) | 23.4±4.3 (24.5) | 24.1±4.3 (25) | 0.535b |
| GDS score (n = 123) | 5.0±3.3 (4) | 5±2.9 (4) | 5±3.4 (5) | 0.840b |
| Body mass index | 26.9±4.9 (26.8) | 23.2±4.4 (21.5) | 27.2±4.8 (27.2) | 0.003b |
| Right calf circumference (n = 121) | 33.7±3.6 (34) | 30.1±2.9 (30.2) | 34.1±3.4 (34.5) | <0.001c |
| SARC-F ≥4 | 63 (50.8%) | 4 (33.3%) | 59 (52.7%) | 0.234d |
| Gait speed (n = 122) | 1.4±0.5 (1.4) | 1.4±0.6 (1.4) | 1.4±0.5 (1.4) | 0.867b |
| SPPB score | 8.8±2.6 (9) | 8±2.8 (9) | 8.9±2.6 (9) | 0.365b |
| Falls in the last month | 23 (18.5%) | 1 (8.3%) | 22 (19.6%) | 0.463d |
| Falls in the last 6 months | 51 (41.1%) | 5 (41.7%) | 46 (41.1%) | 0.968a |
Data expressed as percentage (%), as well as mean±standard deviation (median); aPearsons chi-squared test; bMann-Whitney test; cStudents T test; dFishers exact test; SE ADL, Schwab and England Activities of Daily Living Scale; LED, Levodopa Equivalent Dose; GDS, Geriatric Depression Scale; MMSE, Mini-Mental State Examination; GDS, Geriatric Depression Scale; SPPB, Short Physical Performance Battery.
Table 3
Multivariate analysis for sarcopenia
| Probable sarcopenia | ||
| Predictors | OR (95% CI) | p |
| LED (100 mg) | 0.85 (0.77–0.95) | 0.005 |
| MDS-UPDRS Part III score (10 pts) | 1.5 (1.03–2.2) | 0.032 |
| MMSE score | 0.84 (0.74–0.95) | 0.005 |
| Confirmed sarcopenia | ||
| Predictors | OR (95% CI) | p |
| LED (100 mg) | 0.83 (0.72–0.96) | 0.013 |
| Right calf circumference | 0.69 (0.56–0.86) | 0.001 |
LED, Levodopa Equivalent Dose; MMSE, Mini-Mental State Examination.
Sensitivity, specificity, and ROC analyses and cut-off determination for identification of sarcopenia
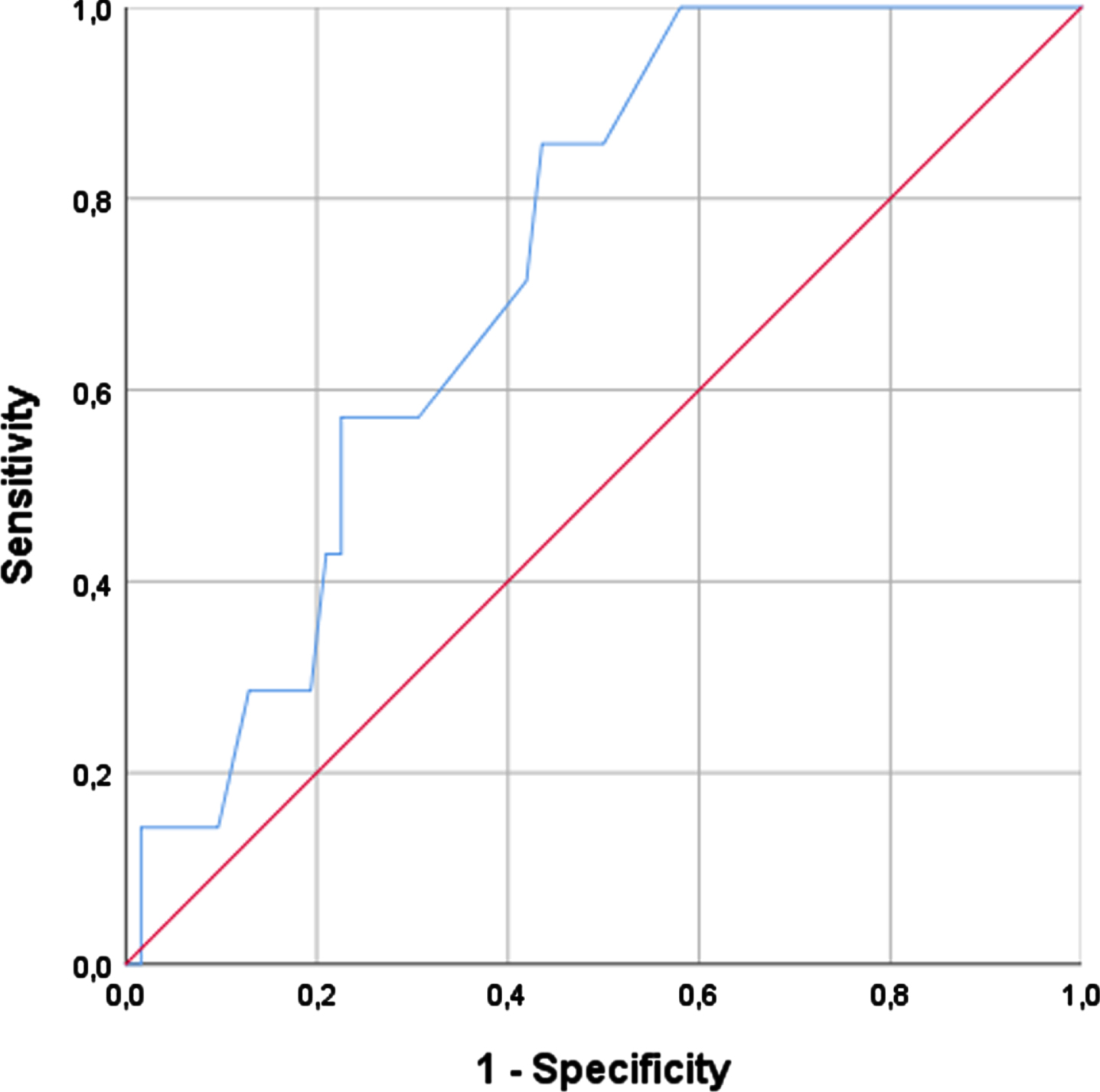
A CC cut-off of 31 cm showed the best balance between sensitivity and specificity in women (sensitivity of 100% and specificity of 78.7%) (AUC = 0.915) corresponding to the cut-off to identify confirmed sarcopenia. The most suitable cut-off regarding men was 34 cm (sensitivity of 85.7% and specificity of 56.4%) (AUC = 0.730). Considering these cut-offs, we also assessed the CC to confirm sarcopenia among all PD patients (wo with low handgrip strength, showing a sensitivity of 91.7% and specificity of 76.9% (Table 4).
Table 4
Sensitivity, specificity, PPV, NPV, +LR and – LR analyses and ROC curves for right CC and SARC-F validation against sarcopenia
| <31 cm in women | <34 cm in men | SARC-F ≥4 | CC <31 cm in women and <34 cm in men* | |
| Sensitivity % | 100 (47.8–100) | 85.7 (42.1–99.6) | 33.3 (9.9–65.1) | 91.7 (61.5–99.8) |
| Specificity % | 78.7 (64.3–89.3) | 56.4 (43.2–69) | 47.3 (37.8–57) | 76.9 (46.2–95) |
| PPV % | 33.3 (22.4–46.4) | 18.2 (12.8–25.2) | 6.3 (2.9–13.3) | 78.6 (57.2–90.9) |
| NPV % | 100 | 97.2 (84.9–99.5) | 86.9 (80.9–91.2) | 90.9 (59.9–98.5) |
| AUC | 0.915 | 0.730 | 0.469 | 0.986 and 0.845 |
| +LR | 4.7 (2.7–8.1) | 2.0 (1.3–3.0) | 0.6 (0.3–1.4) | 4 (1.4–10.9) |
| –LR | 0 | 0.2 (0.04–1.6) | 1.4 (0.9–2.2) | 0.1 (0.02–0.7) |
| Accuracy | 80.8 (64.5–90.4) | 59.4 (46.9–71.1) | 46 (37–55.1) | 84.0 (63.9–95.5) |
*Among patients with probable sarcopenia; PPV, positive predictive value; NPV, negative predictive value; AUC, area under the curve; +LR, positive likelihood ratio; – LR, negative likelihood ratio.
SARC-F showed low accuracy in the diagnosis of sarcopenia. The cut-off ≥4 had the best balance between sensitivity and specificity for confirmed sarcopenia (sensitivity: 33.3%; specificity: 47.3%; AUC: 0.469). These results are also shown in Table 4.
DISCUSSION
This study aimed to estimate the prevalence of probable sarcopenia, confirmed sarcopenia and severe sarcopenia in PD patients with Hoehn and Yahr stage 1 to 3, finding 20%, 9.6%, and 3.2%, respectively. Clinical and sociodemographic features of the study sample were assessed. Lower LED and CC were independently associated with confirmed sarcopenia. Lower LED, MDS-UPDRS Part III score, and MMSE score were independently associated with probable sarcopenia. This study showed that CC was significantly more accurate than SARC-F in sarcopenia screening among PD patients in terms of sensitivity and specificity. We confirmed the validity of optimal cut-offs (women: <31 cm; men: <34 cm) in sarcopenia screening. The CC cut-off for women had excellent accuracy to confirm sarcopenia among PD patients with low handgrip strength.
There are some challenges in trying to estimate the prevalence of sarcopenia in PD patients. First, there is scarce evidence regarding the accuracy of the SARC-F questionnaire to screen patients for sarcopenia in PD patients [22, 23]. Some studies have shown low sensitivity of the SARC-F in parkinsonian patients like our study [23]. Indeed, PD patients who require assistance in walking, have difficulty to get up from a chair, to lift and carry 10 pounds and to climb stairs, as well as experiencing falls, might all be related to parkinsonian symptoms such as rigidity, bradykinesia, postural instability, and orthostatic hypotension. In this sense, SARC-F is not well established as an accurate screening tool for sarcopenia in this population [21, 23]. PD motor symptoms may further interfere with currently used standardized procedures to assess muscle strength. The chair stand test might particularly not be an accurate measure, since the time to get up from a chair is possibly related to bradykinesia and rigidity beyond postural instability instead of muscle function [24]. Therefore, we only used the handgrip strength to assess muscle strength and diagnose probable sarcopenia. Among patients with probable sarcopenia, only those with low muscle quantity according to DEXA were considered to have confirmed sarcopenia, in accordance with current EWGSOP 2 criteria. The sarcopenia prevalence in our sample of PD patients was 9.6%, which agrees with the overall prevalence of sarcopenia in healthy adults aged ≥60 years (10%) in a recent systematic review and meta-analysis of population-based studies. It is worth mentioning that this systematic review found different definitions and diagnostic criteria among studies [2].
Few studies have assessed the prevalence of sarcopenia in PD patients [6]. Methodological dissimilarities, mainly variable definitions of sarcopenia and patient selection methods, hamper comparisons across studies. To date, the prevalence of sarcopenia in PD patients ranges from 6% to 55.8% [6]. In another study conducted by our study group, the prevalence of probable sarcopenia according to EWGSOP 2 criteria was 47.4% [25]. The FTSTS test was used in addition to handgrip strength to assess for probable sarcopenia, which might partially explain the different results [26]. The FTSTS test has been validated by Duncan (2011) to assess risk of falls in this population with excellent retest reliability but was not strongly correlated to lower limb strength in multiple regression analysis. In fact, balance and bradykinesia in people with PD appear to be the most important contributing factors for FTSTS performance [24]. Considering this serious limitation, we decided not to include FTSTS to assess strength, but we used it to assess performance (SPPB) when preparing the current study protocol.
A recent study also conducted in Brazil by da Luz et al. (2021) showed a prevalence of sarcopenia of 21.7% in PD patients according to EWGSOP 2 [23]. Although the prevalence was much higher than in our study, it must be highlighted that patients with more advanced disease (HY 4) were also included in that study. Differently from our study, they used bioelectrical impedance analysis to assess muscle mass, and they used different cut-offs for appendicular skeletal muscle index, which were higher (≤7.7 kg/m2 for men and ≤5.62 kg/m2 for women) than those proposed by the EWGSOP 2. Future studies with standardized criteria for defining sarcopenia are needed to better enable accurate estimations of this disease in PD patients and support interventions aiming at reducing its occurrence and consequent burdens.
Sarcopenia was associated with older age in our study. Although it is acknowledged that many other factors contribute to this condition, ageing is associated with a decline in muscle mass and strength [1]. We also observed decreased body mass index and CC among patients with sarcopenia, the last being independently associated in multivariate analysis for confirmed sarcopenia. The occurrence of weight loss among older patients should indeed prompt concerns related to muscle wasting, and there is a strong correlation between CC and appendicular muscle mass in adults, which is independent of age [25]. Recently, da Luz et al. (2021) proposed the use of CC in addition to SARC-F to screen for sarcopenia in PD. Further studies should assess the accuracy of this anthropometric measure for detecting sarcopenia in PD [23]. However, the SARC-F did not show good accuracy to screen for sarcopenia in our study. In agreement with our study, a recent systematic review and meta-analysis concluded that its low to moderate sensitivity makes it non-optimal to use for sarcopenia screening in older adults [27].
We assessed the association between CC and muscle mass determined by DXA and assessed the value of CC as a substitute marker of muscle mass for sarcopenia diagnosis in PD. Our results showed an association of lower CC with confirmed sarcopenia in PD. Given the sensitivity, specificity, and RO Kawakami C curve analyses, we showed that measuring the CC, which is a fast and inexpensive method, can accurately help to identify PD patients with confirmed sarcopenia in clinical practice, with a cut-off of <31 cm in women and <34 cm in men.
Several prior studies have been conducted with the goal of determining cut-off values for low muscle mass screening measured by CC in various populations and health conditions [28–31]. Thus, different cut-off values of CC by geography and ethnicity can be used to identify decreasing muscle mass. Kim et al. reported that optimal CC cut-offs for 70- to 74-year-old participants were 35 cm (sensitivity 92%, specificity 59%) for males and 33 cm (sensitivity 83%, specificity 50%) for females [31]. A Brazilian study of participants aging 60 to 69 years proposed cut-off ratios of 34 cm for males and 33 cm for females [32].
Our study also found that higher levodopa intake was associated with higher appendicular muscle mass and higher handgrip strength in our sample, indicating that levodopa might be suitable to maintain or preserve strength and muscle mass. Previous studies have examined the influence of levodopa medication on muscle strength in PD. Pedersen et al. found that levodopa had an influence on strength production in PD, and that strength measurement can be used to assess the effectiveness of pharmaceutical treatment [33]. In this sense, Corcos et al. demonstrated that levodopa affects both strength and force development rate, and that changes in strength significantly correlate with changes in contraction rate. Thus, Corcos et al. showed that the discontinuation of antiparkinsonian medicine reduces strength [34]. However, there are scarce studies on the topic. Additional research should concentrate on clinical trials with larger sample sizes and appropriate follow-up periods to investigate if the Levodopa intake is effective on muscle strength in PD. Although not definitive, the summary of evidence to date suggests that appropriate levodopa adjustment according to patients’ motor symptoms is crucial to maintain or preserve strength and muscle mass and might prevent sarcopenia.
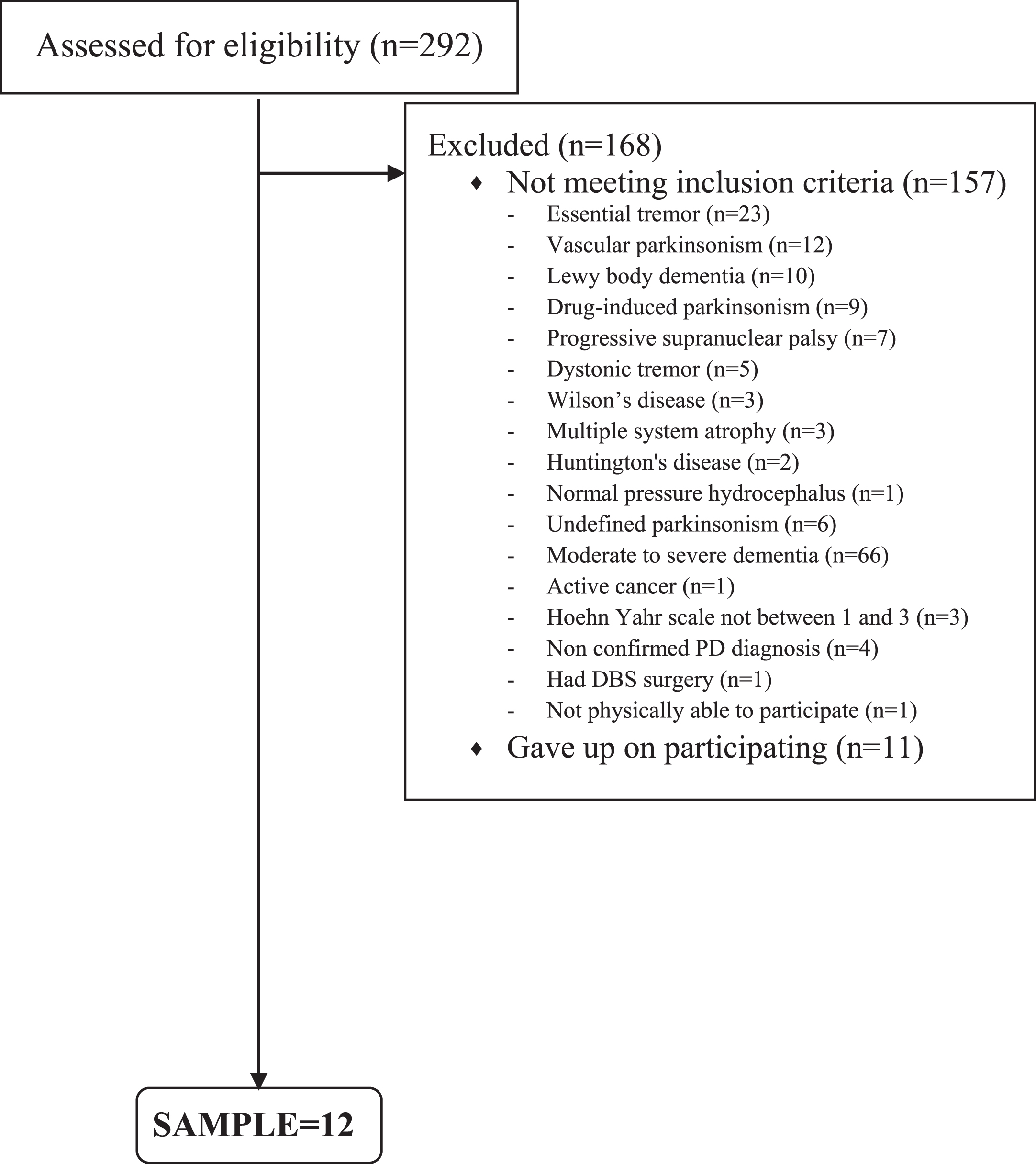
Fig. 1
Flowchart of the recruitment process.
Lower LED suggests either inadequate doses or lower requirement of DOPA, thus suggesting milder disease. Although the evidence that levodopa can improve muscle strength is uncertain [35, 36], many studies have demonstrated the need of using proper dopaminergic treatment to preserve strength and muscle mass and maybe avoid sarcopenia [37, 38]. According to Cioni et al., dopaminergic drugs increase the activity of the muscles in the distal lower limbs [38]. According to Smulders et al., bradykinetic and hypometric spatial aspects of gait and turning improve with dopaminergic therapy [39].
Lower adherence to regular physical activity and decreased physical performance were both associated with probable sarcopenia in this sample. The role of lifestyle factors in developing this condition is already well established and physical inactivity can hasten muscle weakening and favors progression toward functional impairment. These findings support the importance of interventions focusing on regular physical activity to slow these processes and improve physical performance. Also, the association of probable sarcopenia with higher MDS-UPDRS part III score points out that patients with low handgrip strength have more symptomatic disease. Indeed, PD symptoms such as rigidity and bradykinesia might induce low gait speed and impair postural balance, thus contributing to poor physical performance [6, 40]. Considering that lower LED was independently associated with sarcopenia, we might consider that optimizing dopaminergic pharmacological treatment is probably decisive for improving strength and physical performance of PD patients.
Worse cognitive performance as assessed by MMSE was independently associated with sarcopenia in our study. This is consistent with previous studies that not only showed an independent association, but also that sarcopenia is associated with an increased risk of cognitive impairment in the general population, as found in two recent systematic reviews and meta-analyses [41, 42]. This is an issue of particular concern among patients with PD given that this is a neurodegenerative disease that involves progressive decline of cognitive function across different domains.
In addition to aging and physical inactivity, there is a wide range of factors that contribute to sarcopenia development, such as chronic illness and poor nutrition. In this sense, different assessment methods and study proceedings could also identify other variables related to those factors in this sample [1].
We cannot conclude that PD itself does not contribute to sarcopenia for some reasons: firstly, we did not compare our PD sample with a control group. Secondly our sample is small. The considerable disparity between studies could be attributed to diagnostic criteria, muscle mass measuring procedures, differing cut-off values for muscle mass indices for the definition of sarcopenia, and the characteristics and age of recruited PD patients [6]. Several theories regarding sarcopenia have been created through various working groups or associations. A recent systematic review and meta-analysis by Cai et al. found considerable heterogeneity among studies evaluating sarcopenia in PD. They analyzed 10 studies and found that the prevalence of sarcopenia in PD was 29%, but this figure reduced to 17% when only papers with a low risk of bias were considered. Nonetheless, there was no analysis of age subgroups in these authors’ meta-analyses. Also, the disease durations of PD patients were varied among different studies [6]. Another recent systematic review and meta-analysis noticed that PD patients had 3.98 times the prevalence of sarcopenia as controls [43]. Five studies out of nine confirmed low muscle mass using bioimpedance, while one study did not confirm low muscle mass using bioimpedance, DEXA, or any other type of imaging. They also observed significant heterogeneity in the outcomes, and they did not test the effect of the time of PD.
Fig. 2
SARC-F and Confirmed Sarcopenia.
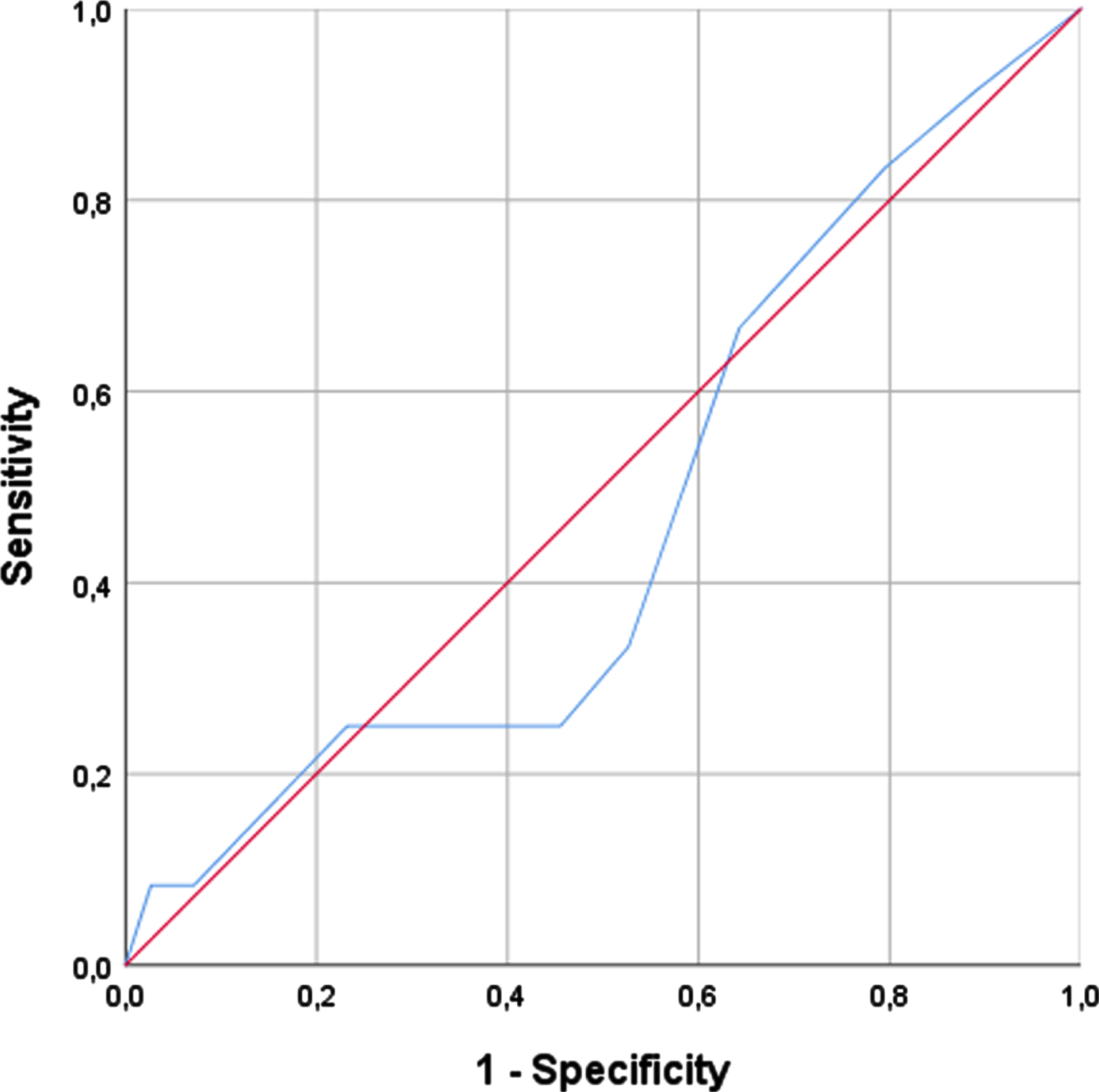
Fig. 3
Right calf circumference and confirmed sarcopenia in women.
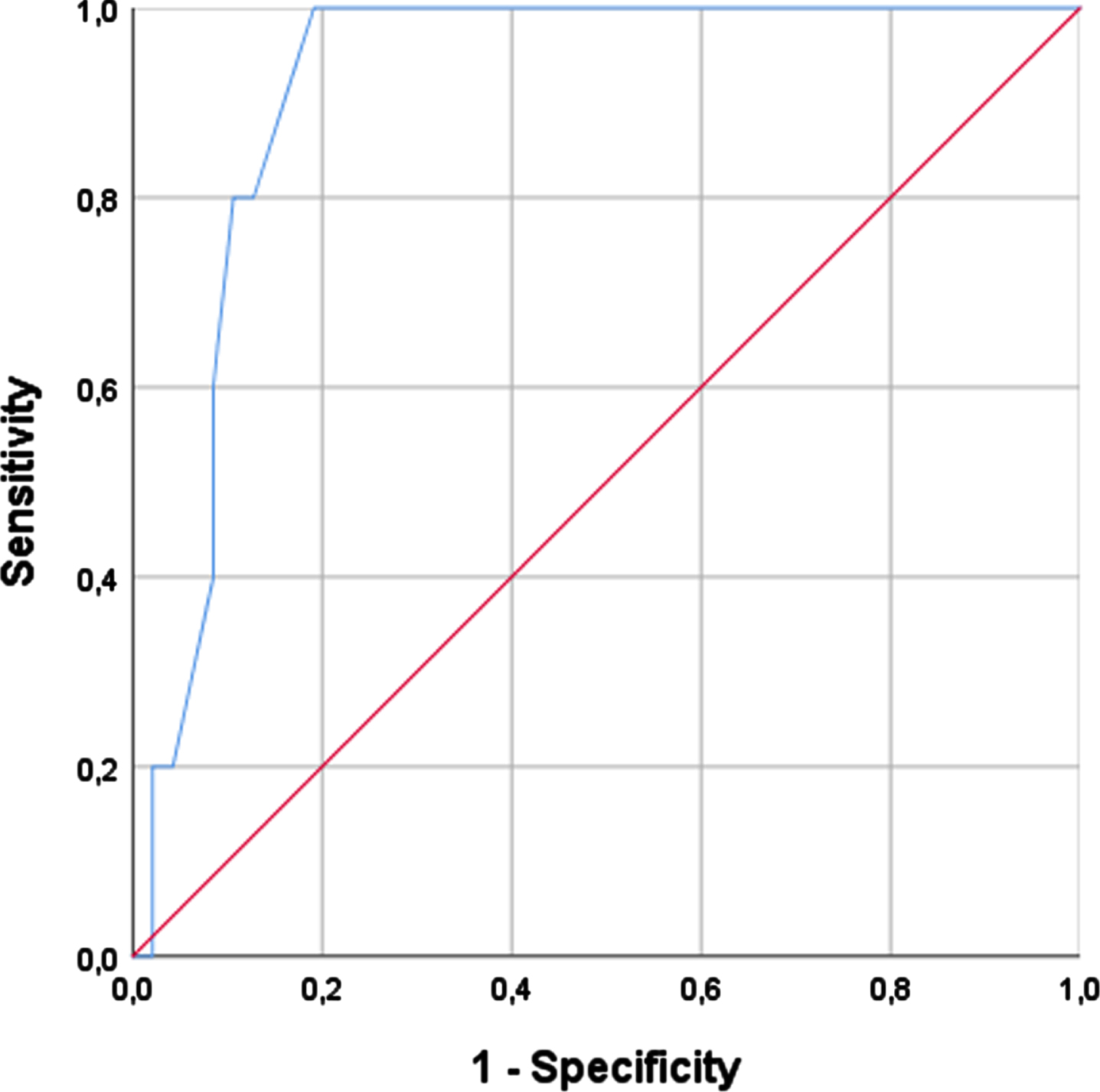
Fig. 4
Right calf circumference and confirmed sarcopenia in men.
The current study has some limitations. First, patients with severe disease (HY 4-5) and who were wheelchair users were not included. Therefore, the prevalence of sarcopenia might have been underestimated in our study. Associations described herein may not be valid among those with more advanced disease. It should also be noted that normality curves and cut-off points for muscle mass and function in PD patients are not yet available. The sarcopenia cut-off points used in this study were based on cut-off points for healthy adults as recommended by the EWGSOP 2. Moreover, there are not any validated methods to date to diagnose probable sarcopenia by measuring lower limb strength in PD patients. The addition of a validated method to measure lower limb strength will probably increase the accuracy to detect sarcopenia in PD patients.
Conclusion
Our study showed a low prevalence of confirmed sarcopenia among PD patients according to the EWGSOP2 using grip strength and DEXA, independently associated with lower LED and lower CC. A better understanding of the interrelationship between sarcopenia and pharmacological treatment for PD may help in improving patients’ long-term outcomes. We propose that healthcare providers introduce measuring CC, which is a quick and inexpensive method to assess for sarcopenia in PD patients. A cut-off of <31 cm in women and <34 cm in men with low handgrip strength showed great accuracy to diagnose sarcopenia in PD patients. We recommend the measurement of CC in patients with low handgrip strength in case DXA is not available. The prevalence and related clinical features in this study are only applicable to independent, mild-mid stage PD without mobility-associated co-morbidities. The CC cut-off relates to this study’s subjects or may be determined by ethnicity. Additional studies including a diverse range of ethnicities and a considerably larger sample size will reveal the ethnicity-specific cut-off value.
FUNDING
One of the authors (Pedro Braga Neto) received funding from the Brazilian National Council for Scientific and Technological Development (CNPq) as research grant funding.
ACKNOWLEDGMENTS
We gratefully acknowledge the patients and their caregivers for agreeing to participate in this study.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
The data supporting the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
[1] | Cruz-Jentoft AJ , Bahat G , Bauer J , Boirie Y , Bruyère O , Cederholm T ((2019) ) Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 48: , 16–31. |
[2] | Shafiee G , Keshtkar A , Soltani A , Ahadi Z , Larijani B , Heshmat R ((2017) ) Prevalence of sarcopenia in the world: A systematic review and meta- analysis of general population studies. J Diabetes Metab Disord 16: , 21. |
[3] | Bloem BR , Okun MS , Klein C ((2021) ) Parkinson’s disease. Lancet 397: , 2284–2303. |
[4] | Kwan P ((2013) ) Sarcopenia, a neurogenic syndrome? J Aging Res 2013: , 791679. |
[5] | Vetrano DL , Pisciotta MS , Laudisio A , Lo Monaco MR , Onder G , Brandi V ((2018) ) Sarcopenia in Parkinson disease: Comparison of different criteria and association with disease severity. J Am Med Dir Assoc 19: , 523–527. |
[6] | Cai Y , Feng F , Wei Q , Jiang Z , Ou R , Shang H ((2021) ) Sarcopenia in patients with Parkinson’s disease: A systematic review and meta-analysis. Front Neurol 12: , 598035. |
[7] | Postuma RB , Berg D , Stern M , Poewe W , Olanow CW , Oertel W ((2015) ) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30: , 1591–1601. |
[8] | Tomlinson CL , Stowe R , Patel S , Rick C , Gray R , Clarke CE ((2010) ) Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord 25: , 2649–2653. |
[9] | Martinez-Martin P , Rodriguez-Blazquez C , Alvarez-Sanchez M , Arakaki T , Bergareche-Yarza A , Chade A ((2013) ) Expanded and independent validation of the Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS). J Neurol 260: , 228–236. |
[10] | De Castro-Costa É , Dewey ME , Uchôa E , Firmo JOA , Lima-Costa MF , Stewart R ((2014) ) Construct validity of the Mini Mental State Examination across time in a sample with low-education levels: 10-Year follow-up of the Bambú cohort study of ageing. Int J Geriatr Psychiatry 29: , 1294–1303. |
[11] | Kim J , Wang ZM , Heymsfield SB , Baumgartner RN , Gallagher D ((2002) ) Total-body skeletal muscle mass: Estimation by a new dual-energy X-ray absorptiometry method. Am J Clin Nutr 76: , 378–383. |
[12] | Kim S , Kim M , Lee Y , Kim BS , Yoon TY , Won CW ((2018) ) Calf circumference as a simple screening marker for diagnosing sarcopenia in older Korean adults: The Korean Frailty and Aging Cohort Study (KFACS). J Korean Med Sci 33: , e151. |
[13] | Bahat G ((2021) ) Measuring calf circumference: A practical tool to predict skeletal muscle mass via adjustment with BMI. Am J Clin Nutr 113: , 1398–1399. |
[14] | Tosato M , Marzetti E , Cesari M , Savera G , Miller RR , Bernabei R ((2017) ) Measurement of muscle mass in sarcopenia: From imaging to biochemical markers. Aging Clin Exp Res 29: , 19–27. |
[15] | Jeong E , Kim M , Won CW ((2020) ) Effects of posture, side and dominant hand on calf circumference measurement in community-dwelling older adults. Geriatr Gerontol Int 20: , 822–827. |
[16] | Wearing J , Konings P , De Bie RA , Stokes M , De Bruin ED ((2020) ) Prevalence of probable sarcopenia in community-dwelling older Swiss people- A cross-sectional study. BMC Geriatr 20: , 307. |
[17] | Tan LF , Lim ZY , Choe R , Seetharaman S , Merchant R ((2017) ) Screening for frailty and sarcopenia among older persons in medical outpatient clinics and its associations with healthcare burden. J Am Med Dir Assoc 18: , 583–587. |
[18] | Roberts HC , Denison HJ , Martin HJ , Patel HP , Syddall H , Cooper C ((2011) ) A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardized approach. Age Ageing 40: , 423–429. |
[19] | de Fátima Ribeiro Silva C , Ohara DG , Matos AP , Pinto ACPN , Pegorari MS ((2021) ) Short physical performance battery as a measure of physical performance and mortality predictor in older adults: A comprehensive literature review. Int J Environ Res Public Health 18: , 10612. |
[20] | Gómez Montes JF , Curcio CL , Alvarado B , Zunzunegui MV , Guralnik J ((2013) ) Validity and reliability of the Short Physical Performance Battery (SPPB): A pilot study on mobility in the Colombian Andes. Colomb Med 44: , 165–171. |
[21] | Ozer FF , Akın S , Gultekin M , Zararsız GE ((2020) ) Sarcopenia, dynapenia, and body composition in Parkinson’s disease: Are they good predictors of disability? A case– control study. Neurol Sci 41: , 313–320. |
[22] | Gould H , Brennan SL , Kotowicz MA , Nicholson GC , Pasco JA ((2014) ) Total and appendicular lean mass reference ranges for Australian men and women: The Geelong osteoporosis study. Calcif Tissue Int 94: , 363–372. |
[23] | da Luz MCL , Pinho CPS , Bezerra GK de A , da Conceição Chaves de Lemos M , da Silva Diniz A , Cabral PC ((2021) ) SARC-F and SARC-CalF in screening for sarcopenia in older adults with Parkinson’s disease. Exp Gerontol 144: , 111183. |
[24] | Duncan RP , Leddy AL , Earhart GM ((2011) ) Five times sit-to-stand test performance in Parkinson’s disease. Arch Phys Med Rehabil 92: , 1431–1436. |
[25] | Santos LP , Gonzalez MC , Orlandi SP , Bielemann RM , Barbosa-Silva TG , Heymsfield SB ((2019) ) New prediction equations to estimate appendicular skeletal muscle mass using calf circumference: Results from NHANES 1999–2006. J Parenter Enter Nutr 43: , 998–1007. |
[26] | Lima DP , de Almeida SB , Bonfadini JC , de Luna JRG , de Alencar MS , Pinheiro-Neto EB , Viana-Júnior AB , Veras SRO , Sobreira-Neto MA , Roriz-Filho JS , Braga-Neto P ((2020) ) Clinical correlates of sarcopenia and falls in Parkinson’s disease. PLoS One 15: , e0227238. |
[27] | Voelker SN , Michalopoulos N , Maier AB , Reijnierse EM ((2021) ) Reliability and concurrent validity of the SARC-F and its modified versions: A systematic review and meta-analysis. J Am Med Dir Assoc 22: , 1864–1876.e16. |
[28] | Kawakami R , Murakami H , Sanada K , Tanaka N , Sawada SS , Tabata I , Higuchi M , Miyachi M ((2015) ) Calf circumference as a surrogate marker of muscle mass for diagnosing sarcopenia in Japanese men and women. Geriatr Gerontol Int 15: , 969–576. |
[29] | Kawakami R , Miyachi M , Sawada SS , Torii S , Midorikawa T , Tanisawa K , Ito T , Usui C , Ishii K , Suzuki K , Sakamoto S , Higuchi M , Muraoka I , Oka K ((2020) ) Cut-offs for calf circumference as a screening tool for low muscle mass: WASEDA’S Health Study. Geriatr Gerontol Int 20: , 943–950. |
[30] | Kim M , Jeong MJ , Yoo J , Song DY , Won CW ((2018) ) Calf circumference as a screening tool for cognitive frailty in community-dwelling older adults: The Korean frailty and aging cohort study (KFACS). J Clin Med 7: , 332. |
[31] | Inoue T , Maeda K , Shimizu A , Nagano A , Ueshima J , Sato K , Murotani K ((2021) ) Calf circumference value for sarcopenia screening among older adults with stroke. Arch Gerontol Geriatr 93: , 104–290. |
[32] | Barbosa-Silva TG , Bielemann RM , Gonzalez MC , Menezes AMB ((2016) ) Prevalence of sarcopenia among community-dwelling elderly of a medium-sized South American city: Results of the COMO VAI? Study. J Cachexia Sarcopenia Muscle 7: , 136–143. |
[33] | Pedersen SW , Oherg B ((1993) ) Dynamic strength in Parkinson ’s disease: Quantitative measurements following withdrawal of medication. Eur Neurol 33: , 97–102. |
[34] | Corcos DM , Chen CM , Quinn NP , McAuley J , Rothwell JC ((1996) ) Strength in Parkinson’s disease: Relationship to rate of force generation and clinical status. Ann Neurol 39: , 79–88. |
[35] | Renee S , Elisabeth P , Niruthikha M , Allyson F , Louise A ((2021) ) People with mild PD have impaired force production in all lower limb muscle groups: A cross-sectional study. Physiother Res Int 26: , e1897. |
[36] | Pelicioni PHS , Pereira MP , Lahr J , Dos Santos PCR , Gobbi LTB ((2021) ) Assessment of force production in Parkinson’s disease subtypes. Int J Environ Res Public Health 18: , 10044. |
[37] | Salenius S , Avikainen S , Kaakkola S , Hari R , Brown P ((2002) ) Defective cortical drive to muscle in Parkinson’s disease and its improvement with levodopa. Brain 125: , 491–500. |
[38] | Cioni M , Richards CL , Malouin F , Bedard PJ , Lemieux R ((1997) ) Characteristics of the electromyographic patterns of lower limb muscles during gait in patients with Parkinson’s disease when OFF and ON L-Dopa treatment. Ital J Neurol Sci 18: , 195–208. |
[39] | Smulders K , Dale ML , Carlson-Kuhta P , Nutt JG , Horak FB ((2017) ) Pharmacological treatment in Parkinson’s disease: Effects on gait. Parkinsonism Relat Disord 3: , 3–13. |
[40] | Lee CY , Chen HL , Chen PC , Chen YS , Chiang PL , Wang CK , Lu CH , Chen MH , Chou KH , Huang YC , Lin WC ((2019) ) Correlation between executive network integrity and sarcopenia in patients with Parkinson’s disease. Int J Environ Res Public Health 16: , 24. |
[41] | Chang KV , Hsu TH , Wu WT , Huang KC , Han DS ((2016) ) Association between sarcopenia and cognitive impairment: A systematic review and meta-analysis. J Am Med Dir Assoc 17: , 1164.e7–1164.e15. |
[42] | Peng TC , Chen WL , Wu LW , Chang YW , Kao TW ((2020) ) Sarcopenia and cognitive impairment: A systematic review and meta-analysis. Clin Nutr 39: , 2695–2701. |
[43] | Ponsoni A , Sardeli AV , Costa FP , Mourão LF ((2023) ) Prevalence of sarcopenia in Parkinson’s disease: A systematic review and meta-analysis. Geriatr Nurs 49: , 44–49. |



