Dental and oral implications of prescribed Oral Nutritional Supplements for disease related malnutrition- A systematic review
Abstract
BACKGROUND AND OBJECTIVES:
The use of Oral Nutritional Supplements (ONS) is widespread among patients with long- and short-term medical conditions. Although ONS serve an important purpose in the management of malnutrition, their effect on the oral hard and soft tissues is not well understood. The aim of this article is to conduct an analysis of the available literature relating to ONS and their impact on the oral environment.
METHODS:
This study was conducted following the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines adapted by Liberati. The PICO question is as follows:
Population: Individuals (both children and adults)
Intervention: Use of Oral Nutritional Supplements
Comparison: Individuals not taking Oral Nutritional Supplements
Outcome: Increased risk of oral disease (specifically dental caries, periodontal disease or candida)
The research question was “Are people who take ONS at higher risk of oral diseases than the general population?”
Eligibility criteria
The results obtained from the literature search were filtered, according to these inclusion and exclusion criteria:
Inclusion criteria
• Individuals were prescribed Oral Nutritional Supplements
• All studies were included, including, case–control, cohort, cross-sectional or case studies.
• Studies were included if they directly compared the association between ONS and oral health i.e. Effect of ONS on the oral environment had to be one of the aims of the study
• Studies on human subjects or in vitro experiments
• Published in English language
• Studies from 1960 to the present day
Exclusion criteria
• Studies not in English
• Review articles and case series were excluded
• Studies including the use of other nutritional supplements such as vitamin or herbal supplements were not included.
• Other supplemental feeding methods such as enteral or parenteral feeding were not examined
4 databases were searched: Medline (via Ebsco), Embase, Web of Science Core Collection, Google Scholar. Risk of bias was assessed using the ROBINS-I tool.
RESULTS:
A total of 58 records were identified through databases and searching bibliographies. 50 publications were excluded from the review, based on study title and abstract. The full text of eight articles were assessed for eligibility. No article answered the primary aim of the systematic review. Three articles discussed the secondary aim of the systematic review and these were included in the qualitative systematic review.
The main outcome of the first study showed that the ONS had higher cariogenic potential than milk due to it’s higher acidogenicity. However, there was no statistical difference in dentine demineralisation and no significant difference in viable micro-organisms present. The main outcome of the second study showed that Escherichia coli, Staphylococcus aureus and C. albicans all grew at room temperature in the dairy-based ONS collected, and that C. albicans also grew in the juice which was milk protein-free and lipid-free. The third study showed that ONS were “potentially cariogenic” on enamel.
DISCUSSION:
Two studies were deemed as being at “low” of bias, however another study was deemed to be at “serious” level of bias. All studies stressed the possibility of dental disease caused by oral nutritional supplements, however there is not enough research available to imply causation. Due to the high sugar content of these supplements, and the known dental implications of this, it would be beneficial to carry out more research into this area.
1Introduction
1.1Oral Nutritional Supplements
Oral Nutritional Supplements (ONS) or Food for Special Medical Purposes (FMSP) are a form of oral nutrition therapy, “developed to provide energy and nutrient-dense solutions that are provided as ready to drink liquids, cremes or powder supplements that can be prepared as drinks or added to drinks and foods” [1]. They can be provided in the management of patients with a “limited, impaired or disturbed capacity to take, digest, absorb, metabolise or excrete ordinary food or certain nutrients contained therein, or metabolites, or with other medically-determined nutrient requirements, whose dietary management cannot be achieved by modification of the normal diet alone” [1, 2].
Reasons for prescribing ONS are listed in Table 1 below [3]
Table 1
Criteria for prescribing oral nutritional supplements (ONS) in the community setting, reprinted with permission from Kennelly et al. [3]
| Criteria for | Health reasons |
| ONS prescribing | |
| (a) Presence of disease related malnutrition or nutritional risk [4] | |
| (b) Patient not consuming adequate food to meet energy and protein requirements according to nutritional assessment by a dietitian or trained health professional [4] | |
| (c) Patient has an active disease state (e.g. renal disease, liver disease, respiratory disease) or is pre- or post-operative with increased nutritional requirements [4, 5] | |
| (d) Patient’s ability to absorb food is affected by disease (e.g. inflammatory bowel disease, bowel fistulae, cystic fibrosis) and pre- or post-operative state (e.g. total gastrectomy) [6] | |
| (e) Pre- and post-operative undernourished patients (e.g. hip fracture or orthopaedic surgery) [7] | |
| (f) Anorexia and or cachexia as a result of chronic disease or treatment [8, 9] | |
| (g) Patient has problems with eating, drinking, swallowing, including dysphagia, dental problems, sore mouth, dry mouth [10] | |
| (h) Clinically diagnosed depression where there is anorexia and poor motivation to eat [7] | |
| (i) Patient has mobility problems affecting ability to obtain, prepare or consume foods (see social reasons) | |
| (j) To prevent, or improve the healing of, pressure ulcers [7] | |
| (k) Early and moderate dementia to ensure adequate energy and nutrients [7] | |
| Social reasons [10] | |
| (l) Financial difficulties affecting ability to buy food | |
| (m) Difficulties cooking/shopping/preparing food. | |
| (n) Living alone, or eating majority of meals alone and poor motivation to eat. |
It can be seen from Table 1 that there are some circumstances where the consumption of ONS will only be short term, e.g. post-operatively after a gastrectomy, however individuals with chronic conditions such as cystic fibrosis or renal disease. Therefore, any effects ONS may have on the oral environment will most likely be dependent on amount, type and duration of ONS that would be prescribed and consumed.
A recent study in Ireland found that the majority of people taking ONS were female (58.2%), the median age was 76 years and 18.7% were in residential care [11]. This is similar to global studies, with the majority (72.5%) of ONS consumers being female, with a mean age of 81.9 years and ONS being prescribed to 13.9% of nursing home residents [12, 13]. This varies from country to country, with only 1.2% of nursing home residents receiving ONS in Portugal, compared to 43.2% in Turkey [12]. Another study, from the Republic of Ireland found that the majority of patients they examined were prescribed ONS for more than 6 months without review [3].
The benefits of ONS for malnourished individuals are well-established [14, 15] and include reduced mortality, reduction in number of hospital admissions and duration of hospital stays in people who are malnourished [16]. Recent studies have shown protein-rich ONS as a promising treatment for sarcopenia [17]. They have also been shown to improve patients’ quality of life and can result in a gain in quality-adjusted life years (QALYs) [18]. Despite these clear benefits, there have been some adverse effects reported, such as nausea and diarrhoea [14], and concerns have been raised about the cost-effectiveness of ONS [19]. Also of note is the sugar content in ONS, which can be significant. A recent study showed that some of the most common ONS on the market can contain up to 35 g of sugar per 200 ml whole milk reconstitution [20].
1.2Oral disease
Oral disease includes all diseases of the mouth, both hard tissues (teeth) and soft tissues (mucosa including gingivae). The Global Burden of Disease Study 2017 found that oral diseases were the most common diseases found in mankind, affecting close to 3.5 billion people worldwide [21]. The most common of these was dental caries (“tooth decay”) which affects approximately 2.3 billion people with permanent teeth and the deciduous teeth of 530 million children. Other common oral diseases include periodontal (“gum disease”) and oral candidiasis (“thrush”). It is recognised that oral diseases can impact upon people’s quality of life and well-being, tools such as the Oral Health Impact Profile (OHIP) [22, 23]. Untreated dental disease can result in pain, discomfort and affect an individual’s physical functions such as chewing, smiling and talking and impact upon the individual’s social roles [24]. Elderly people with dental disease are at increased risk of oral frailty, which can be defined as decline in oral function which impacts an individual’s ability to chew, speak and swallow [25]. This in turn can lead to an increased risk of frailty, sarcopenia, disability, and mortality [25].
Dental caries is the localised destruction of dental hard tissues (enamel and dentine) by acidic by-products from the bacterial fermentation of free sugars [26]. This is presented in a simplified visual format in Fig. 1. These sugars can come in the form of added monosaccharides (e.g. glucose (dextrose) and fructose) and disaccharides (e.g. sucrose, lactose and maltose) or sugars naturally present in honey, syrups, fruit juices and fruit juice concentrates [27]. Intake of dietary sugars is the most important risk factor for dental caries, and both the frequency and the amount of sugars consumed are important in caries development [28]. In relation to ONS, many are described as “sip-feeds” in that they should be “sipped” on frequently throughout the day, which will result in increased exposure to any sugars contained. In Ireland, very high-energy sip feeds are the most frequently dispensed type (45% of the cohort examined) [11].
If carious lesions progress, they can lead to cavitation resulting in pain and discomfort. If left untreated, caries can eventually result in infection, sepsis and tooth loss. Exposure to fluoride can limit disease progression as fluoride promotes remineralisation of the dental hard tissues [26]. There is a high prevalence of dental caries amongst old-age populations, with the 2009 UK Adult Dental Health Survey reporting that 27% of adults aged 65–74 years had evidence of dental caries, and this figure increased to 40% for those aged 75–84 years [29, 30].
Fig. 1
A simplified visual representation of the multifactorial aetiology of dental caries. Dietary sugars are metabolized by bacteria present in dental biofilms, generating acidic by-products that can lead to caries. The fourth factor that this disease process depends on is time, i.e. that it will take time for this process to occur.
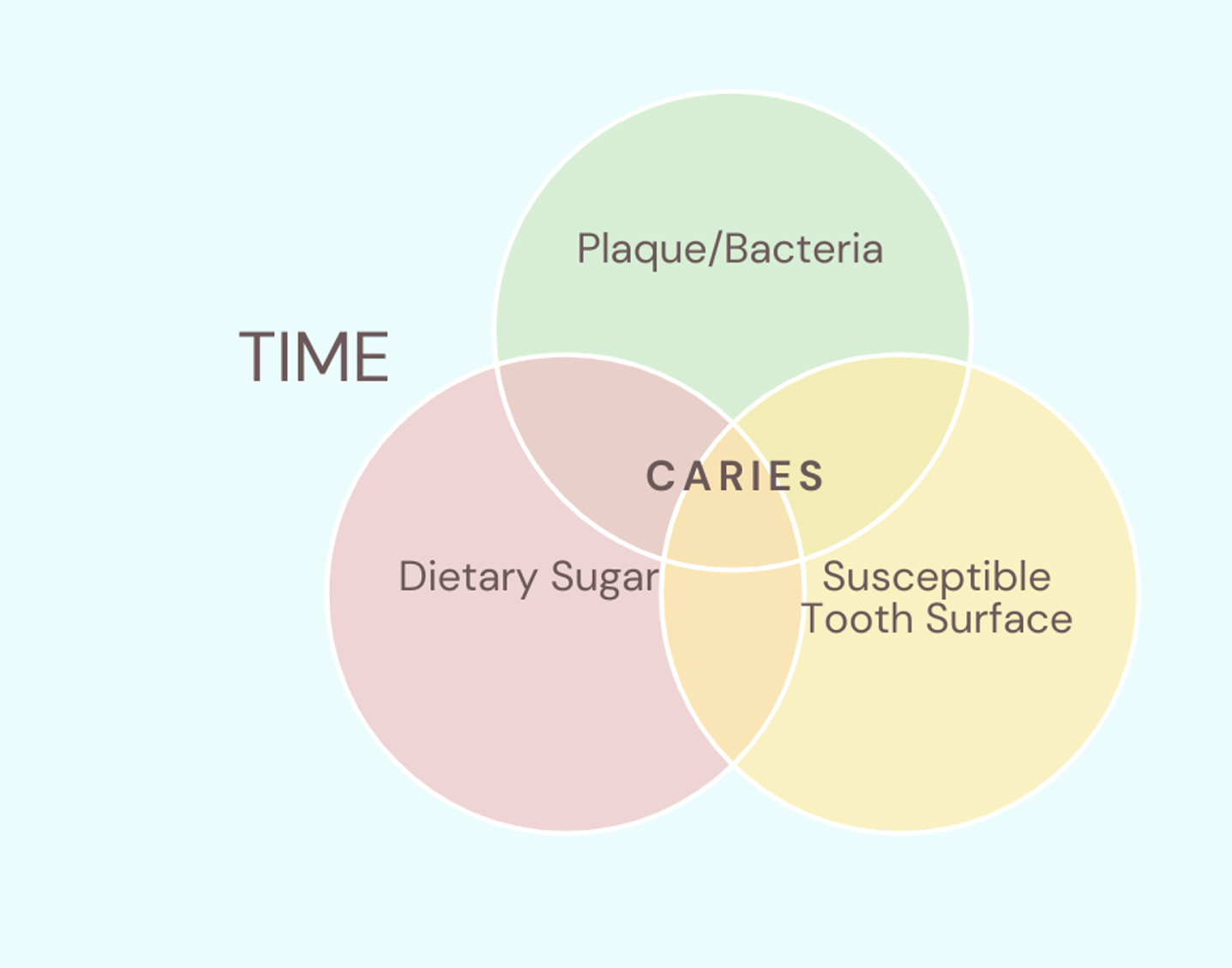
Fig. 2
Patient with periodontal disease. Note erythema around gingival margin, loss of interdental papillae, recession and tooth loss associated with this disease (Photo credit: Prof. Anthony Roberts, UCC).
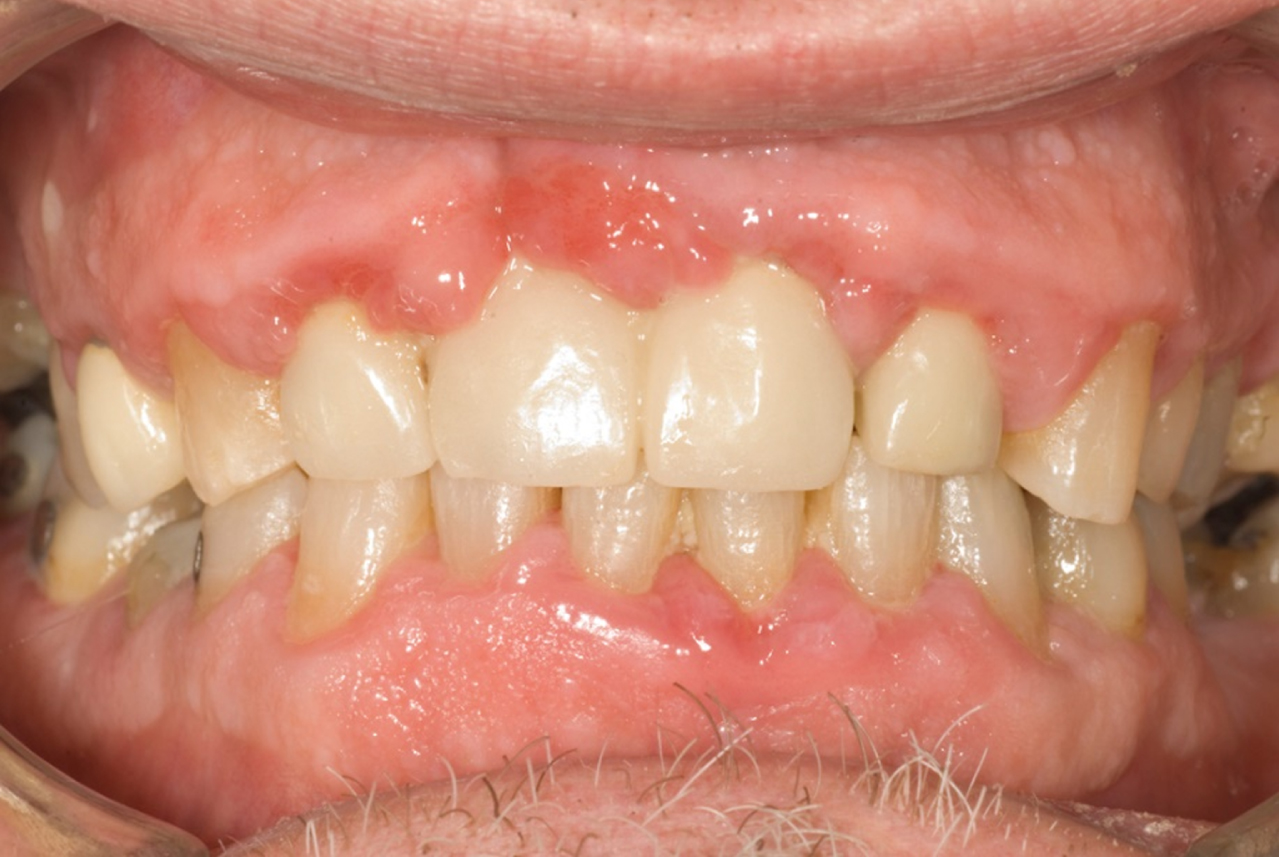
Periodontal diseases (namely, gingivitis and periodontitis) are chronic inflammatory conditions of the tissues surrounding the teeth [26]. Gingivitis, which may be seen clinically as red, swollen gums which bleed on probing, is considered to be reversible when adequate oral hygiene measures are implemented and maintained [31]. Periodontitis involves the destruction of the supporting structures of the teeth, which includes the periodontal ligament, bone and soft tissues. Although gingivitis must precede periodontitis, not all cases of gingivitis progress to periodontitis and it can be said to have a site and subject predilection [32]. Similar to dental caries, the prevalence of periodontitis increases with age, and it is a major cause of tooth loss in older adults [33]. Other risk factors for periodontal disease include being of the male gender and genetic conditions, such as Down syndrome [34–36]. Smoking has also been proven to lead to an increase in periodontal disease rates and a decrease in success rates following periodontal treatment [37, 38]. Sugar has also been suggested as a risk factor: analysis of 2437 young adults found that “A high frequency of consumption of added sugars is associated with periodontal disease, independent of traditional risk factors, suggesting that this consumption pattern may contribute to the systemic inflammation observed in periodontal disease” [39].
The European Federation of Periodontology discussed in a 2016 workshop that dental caries and periodontal disease share common genetic, aetiological and environmental factors, and that the prevention and treatment of both disease entities can share similar pathways [40, 41].
Oral candidiasis, commonly known as ‘thrush”, is an opportunistic fungal infection of the oral cavity. The main causative agent, Candida albicans, is a commensal organism, generally regarded as a normal and harmless member of the oral microbiome in humans. However, changes in the host microenvironment can cause it to become pathogenic [42–44]. A key virulence attribute of Candida is its ability to adhere to the host surface, be that the oral epithelium or surfaces of intra-oral prosthetic devices e.g. dentures, orthodontic appliances [44]. Local risk factors for oral candidiasis include salivary dysfunction, poor denture hygiene and ill-fitting dentures, and topical corticosteroid treatment [42]. Systemic factors include broad spectrum antibiotics, immunosuppressive therapy and intake of dietary carbohydrates [42, 45, 46].
Therefore, it can be seen that high sugar consumption is cited as a risk factor in these three common oral diseases. Due the high sugar content of many ONS, and due to the fact that they are often prescribed as “sip feeds” to be taken throughout the day, a systematic review was carried out to investigate if there are any studies examining if ONS result in a higher prevalence of dental disease.
2Review
2.1Protocol
This study was conducted following the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines adapted by Liberati [47]. The PICO question was as follows:
Population: Individuals (both children and adults)
Intervention: Use of Oral Nutritional Supplements
Comparison: Individuals not taking Oral Nutritional Supplements
Outcome: Increased risk of oral disease (specifically dental caries, periodontal disease or candida)
The research question was: “Are people who take ONS at higher risk of oral diseases than the general population?”
The primary aim of this review was to establish if the use of ONS leads to an increase in oral disease levels.
The secondary aim was to search for any studies on the effect of ONS on oral hard and soft tissues, or on oral microbiota.
Eligibility criteria
The results obtained from the literature search were filtered, according to these inclusion and exclusion criteria:
Inclusion criteria
• Individuals were prescribed Oral Nutritional Supplements
• All studies were included, including, case–control, cohort, cross-sectional or case studies
• Studies were included if they directly compared the association between ONS and oral health i.e. Effect of ONS on the oral environment had to be one of the aims of the study
• Studies on human subjects or in vitro experiments
• Published in English language
• Studies from 1960 to the present day
Exclusion criteria
• Studies not in English
• Review articles and case series were excluded
• Studies including the use of other nutritional supplements such as vitamin or herbal supplements were not included
• Other supplemental feeding methods such as enteral or parenteral feeding were not examined.
2.2Information sources
The searches were conducted in the following electronic databases: Medline (via Ebsco), Embase, Web of Science Core Collection, Google Scholar. These databases were selected due to recommendations for optimal searches in systematic reviews [48]. The bibliographies of relevant articles were hand- searched to identify any additional studies that may have not been captured by the digital searches.
2.3Search strategy and selection criteria
The following search terms were used ““Dental health” “periodont*” “caries” “dental” “candida” AND “oral nutritional supplements”. An example of a search strategy in included in appendix 1. The search was last run on 14/11/2021. Two investigators (N.C., F.O’L.) independently reviewed titles and abstracts to determine eligibility for inclusion. Full articles were obtained for the identified titles and those which met the selection criteria were included. Differences of opinion of the two investigators about study eligibility were resolved by discussion with a third author (M.H.).
2.4Risk of bias
For each of the studies, a risk of bias assessment was performed using the ROBINS-I tool for assessing risk of bias in non-randomised studies [49]. Different items are evaluated as described in the ROBINS-I checklist. The items include questions asking for adequate randomization, allocation to appropriate comparison groups and the accounting for confounding and modifying variables, among others. Each item is rated with as Low, Moderate, Serious or Critical risk of bias, or that “no information” was given on which to base a judgement about risk of bias.
3Results
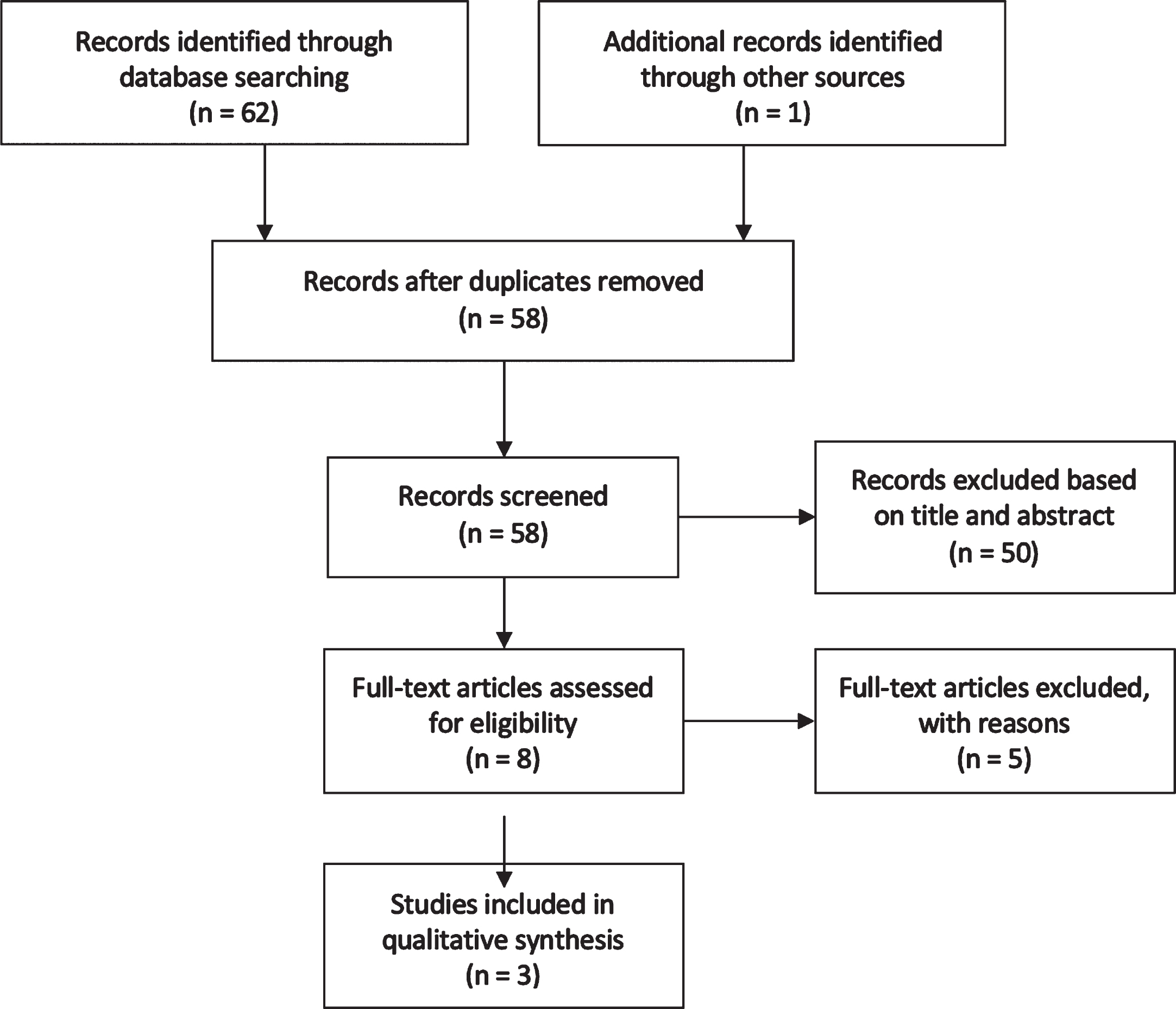
The search resulted in 20 publications from Medline (via Ebsco), Embase, and Web of Science Core Collection. The first 200 results from Google Scholar were analysed [48] of which 42 were deemed to potentially be relevant. Therefore, there was a total of 62 records identified through database searching. One more publication was identified by searching bibliographies. After duplicates were removed, and based on information provided in the study title and abstract, 50 publications were excluded from the review. The full text of eight articles were assessed for eligibility. No article answered the primary aim of the systematic review. Three articles discussed the secondary aim of the systematic review and these were included in the qualitative systematic review. A flow chart of the search results can be seen in Fig. 3.
Fig. 3
Flow Chart Indicating the Number of Records Identified and Included in Systematic Review on ONS and Oral Health.
[One study mentioned that certain ONS can cause mouth drying [50], however this study involved a comparison of casein and whey rich ingredients, as opposed to ONS’ effect on the oral environment and so was not included in the systematic review.]
3.1Study characteristics
Two of the studies [51, 52] were in vitro studies and the third study was an in vivo study [53]. The first study [51] examined the cariogenicity of a milk-based dietary supplement that is distributed to older adults in Chile. The second study [52] investigated if oral microorganisms can proliferate in thickened drinks including ONS. (The second aspect of this study examined lemon-flavoured thickened drinks, but as these did not fit into the inclusion criteria, this is not included in the qualitative review). The third study [53] measured cariogenic potential in vivo utilising intraoral plaque telemetry. As the studies are heterogenous in nature, a meta-analysis was not possible and they will be discussed separately.
3.2Study results
Study No.1: Castro et al, 2019
The aim of this study was “to evaluate the cariogenicity of a milk-based drink intended for older adults that was used as part of a governmental initiative in Chile to improve their nutritional conditions”. The study used a previously validated experimental caries model with bio-films of Streptococcus mutans. 4 items were tested:
1. Skim milk
2. Whole milk
3. Milk-based drink (“Bebida Láctea Anos Dorados”, Calo, Osorno, Chile)
4. Milk-based drink with sucrose (as people commonly add sucrose to these supplements)
The composition of the milk-based drink (Appendix 2) is comparable to other Oral Nutritional supplements [40] and is included in Appendix 2.
The method involved the use of bovine dentine (the main hard dental tissue found on root surfaces) slabs, combined with salivary pellicle from healthy volunteers, inoculated with streptococcus mutans (the main cariogenic bacteria). After 24 hours of biofilm growth, dentine slabs were exposed to the different treatments 3 times per day (at 8 : 30 am, 12 : 30 pm, and 4 : 30 pm) for 5 min. After each treatment, biofilms were washed three times in 0.9% NaCl, and relocated to a medium containing well. Culture medium was changed twice per day before the first (at 8 am) and after the last treatment. The samples were tested for biofilm acidogenicity, dentine demineralisation and viable microorganisms present. The results showed that the milk-based drink had higher cariogenic potential than milk (higher acidogenicity). However, there was no statistical difference in dentine demineralisation and no significant difference in viable micro-organisms present.
This study concluded that the tested milk-based drink used as a dietary supplement for older adults in Chile shows a potential risk for root caries, higher than whole milk. The authors also stressed the importance of testing the cariogenic potential of the dietary supplements available to the elderly population.
Study No.2: Jung et al. 2019
The aim of this study was to investigate in vitro microbial survival or proliferation in ONS. In this study, 4 drinks were tested:
• A strawberry-flavoured dairy drink containing sucrose and milk proteins (Fortimel)
• A vanilla-flavoured dairy cream containing sucrose and milk proteins (Forticreme)
• A cherry-flavoured juice containing sucrose, soya proteins, and pea proteins hydrolysate (Provide Xtra)
• A sucrose-free dairy cream for diabetic patients containing fructose and milk proteins (Fresubin DB)
Seven samples of part open ONS were collected from the Geriatric Ward of Nice University Hospital to verify if they were contaminated after opening and consumption. They had been open for less than 2 hours. They were collected by nurse aides, transported at 4°to the laboratory and immediately inoculated onto Petri dishes. 100μL of ONS were diluted in 900μL of saline water and this 1-mL inoclulum was plated onto sheep blood agar plates. Plates were incubated for 5 days in microaerophilic conditions, adapted to oral microflora, to replicate the oral environment in vivo.
Results were expressed as a qualitative evaluation of the rate of ONS contamination, ranging from – to+++, based on Colony Forming Units (CFUs) numeration on Petri dishes. The samples were tested for 3 micro-organisms: Escherichia coli, Staphylococcus aureus and C. albicans. Qualitative evaluation of microbial growth in the different ONS showed that E. coli, S aureus and C. albicans all grew at room temperature (20° C) in dairy drinks and creams. C. albicans also grew in the juice which was milk protein-free and lipid-free.
The study concluded that ONS are similar to some bacterial and fungal culture media, and may pose a risk of iatrogenic infection for people who consume these.
The degree of contamination was not bound to the type of ONS (dairy ONS vs. juice) but to the contamination of the product by the oral flora of the patient-both types contributed to preserving the viability of some microaerophilic oral strains.
The authors also concluded that ONS could be a source of contamination to other body sites and could increase the risk of aspiration pneumonia.
Study No.3: Stillhart et al., 2021
The aim of this study was to evaluate the cariogenic potential of ONS using intraoral plaque telemetry.
Ten ONS were tested on five healthy volunteers (having no active caries and no medications). A test electrode in a partial denture recorded the plaque-pH values measured for 30minutes. Participants were requested to refrain from toothbrushing and oral hygiene measures for at least 3–7 days in order to allow dental biofilm to build up. The oral fluid was neutralised by chewing on paraffin wax. The participants were then given the ONS and held the solution in the mouth for 2 minutes. pH measurements were recorded for 30 minutes. A rinse with water was then carries out, and participants chewed on paraffin again to neutralise the pH and the saliva. A control solution of 10% sugar was then administered under the same conditions as the ONS. Relative cariogenicity (RC) was expressed as a mean+/– standard deviation. The AuCp (area under the curve) ranged between 6.02±4.34 and 30.57±14.03.
This study showed that all the tested ONS were potentially cariogenic on enamel.
3.3Risk of bias assessment
Different items are evaluated as described in the ROBINS-I checklist for assessing risk of bias in non-randomized studies of interventions. The items include questions asking about adequate randomization, allocation to appropriate comparison groups and the accounting for confounding and modifying variables, among others. Each item is rated with as Low, Moderate, Serious or Critical risk of bias, or that “no information” was given on which to base a judgement about risk of bias. Two of the studies [51, 53] were classified as being at low risk of bias, based on the domains described in the ROBINS-I tool. The study by Jung et al. was classified as being at serious risk of bias. The checklist is presented in Table 2.
Table 2
Risk of Bias assessment
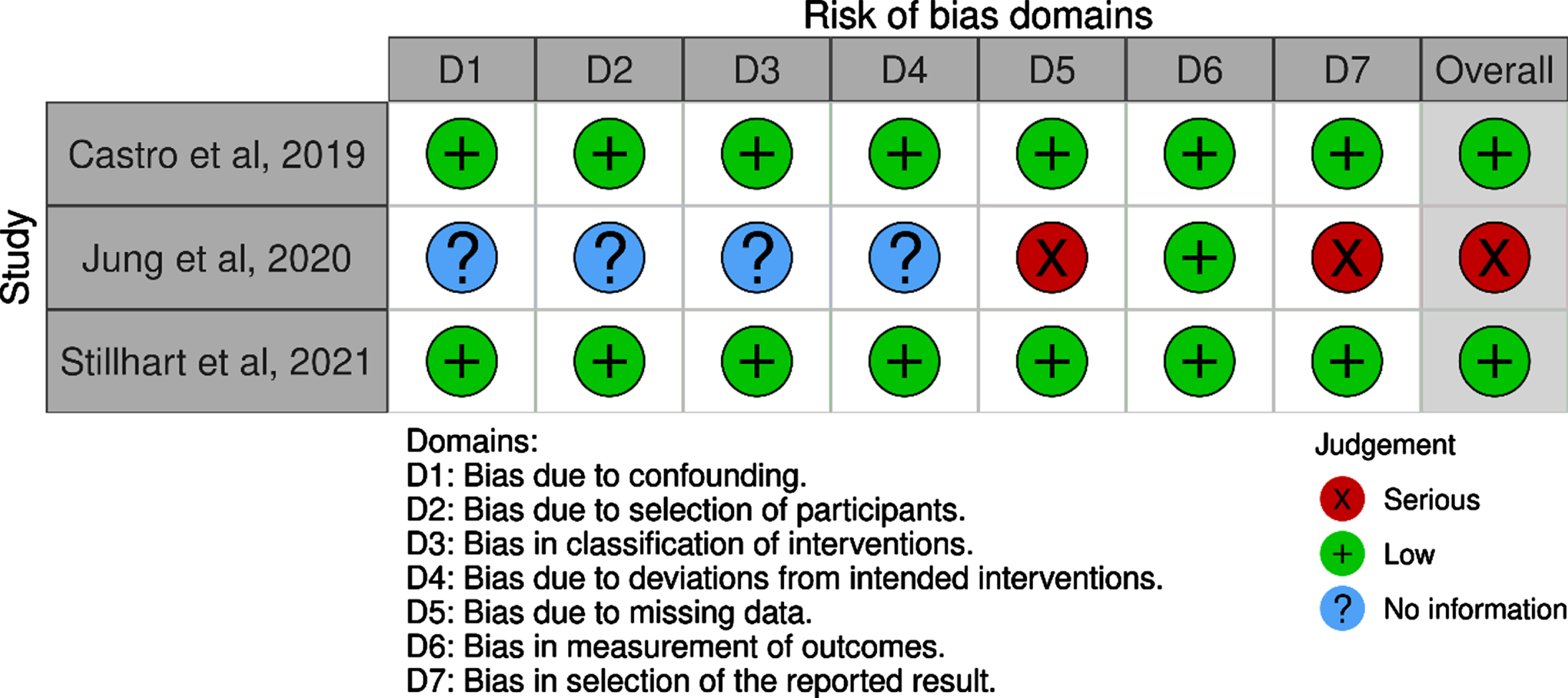 |
4Discussion
As far as the authors are aware, this is the first systematic review into the impact of oral nutritional supplements on the oral environment. The goal of this systematic review was to critically evaluate the literature on the impact that ONS have on the oral environment. The principal finding of this systematic review is that, apart from the recent study by Stillhart et al., there is a lack of high quality research into the effects (if any) that oral nutritional supplements have on the oral environment. The study by Castro et al. showed that these supplements may have higher cariogenic potential than milk due to higher acidogenicity, but failed to find a statistical difference in dentine demineralisation and in viable micro-organisms present. The study by Stillhart et al. was important in that it looked at cariogenic potential in vivo, however the low sample size is a limitation of this study. The study by Jung et al. showed that certain microorganisms (Escherichia coli, Staphylococcus aureus and C. albicans) can grow at room temperature in dairy drinks, however it does not investigate if there is a statistically significant difference in microoganism growth between the supplements and whole milk. All three studies concluded that ONS have the potential to be cariogenic.
It would be difficult, if not impossible, to say if the presence of caries in a person taking ONS is due solely to the consumption of these due to the fact that most people who consume ONS will have comobidities-polpharmacy, poor diet, drug induced xerostomia and so on. However, it is not unreasonable to surmise that the use of these ONS would increase a patient’s risk of dental disease. Therefore, a well-designed clinical study looking at different ONS and their cariogenic potential, while taking into account confounding factors, would be beneficial to further our knowledge on this.
The potential contamination and re-contamination of a patient’s oral environment with micro-organisms such as E. coli, S. aureus and C. albicans is cause for concern as they are implicated in conditions throughout the oral environment (e.g. denture stomatitis [54]) and throughout the body (e.g. aspiration pneumonia [52]). It would be beneficial to carry out further information in this area, using different brands of ONS.
There is a bidirectional relationship between oral health and ONS. One of the main reasons cited for prescribing ONS in malnutrition is “poor dental health”, “poor dentition”, including “missing or broken teeth or poor fitting dentures”, “dental caries or mouth pain” [55–57]. However, if what we are prescribing to treat this problem, i.e. ONS, is actually exacerbating and compounding the problem, it would be wise to reassess our prescription habits. The development of oral frailty, as mentioned in the introduction section, can lead to more serious systemic complications, including sarcopenia, disability and death [25]. Therefore, treatment of oral frailty should be concentrated on preventing any further exacerbation of dental disease. An interesting case of a 90 year old man with dementia was described by Tan et al. [58]. In this case, the man had “10 missing teeth, 9 dental caries, an abscess in a lower molar, and 3 teeth broken at gum level” and was receiving ONS to help with his malnutrition. The team recommended “nutritional and dental reconciliation”, recommending from the dental point of view; symptomatic relief and an aggressive oral hygiene protocol for prevention of further oral disease, and from the nutritional aspect; prescribed snacks with less decay-causing food such as cheese and milk, close supervision and cueing at mealtimes. This commendable approach to patient management should be replicated when at all possible. In nursing homes in particular, oral hygiene is an issue, with one study finding that the oral hygiene of residents is “insufficient” [59] and another stating that “dental evaluations were infrequent and poorly documented in patient charts” [60].
It is the authors’ belief that there is need for increased interprofessional communication in order to optimise both the general and dental health of an individual. This is in-keeping with the Academy of Nutrition and Dietetics’ position that “nutrition is an integral component of oral health. The Academy supports integration of oral health with nutrition services, education, and research. Collaboration between dietetics practitioners and oral health care professionals is recommended for oral health promotion and disease prevention and intervention” [61]. The ESPEN also recommends “dental examination, oral and general health assessment” and recommended dental treatment treatment or salivary substitutes, in order that “Potential causes of malnutrition and dehydration shall be identified and eliminated as far as possible” [62]. An article regarding dietary therapy in chronically sick children and dental considerations of this recommended that “dental health professionals have a background understanding of the dietary treatment of these diseases so that advice to minimize caries does not contradict essential dietary therapy” and also recommended that “dental health professionals liaise effectively with the dietitian and pediatrician to safeguard the oral health of these patients” [63]. Therefore, the use of ONS is certainly not contradicted, even in cases of poor oral health, but a balance should be struck between managing malnutrition and prevention of further oral disease.
A pilot collaborative program between the two professions was carried out at New York University with teaching of “oral health for dietetic interns and nutrition assessment for pediatric dentistry residents” and this had a positive response from both sets of participants [64]. It may be beneficial to consider implementing this in nutrition/dietetic and dental programs in future. The European Federation of Periodontology (EFP) also recommended that, to prevent caries and periodontal disease, oral health education should target (amongst others) care home workers and other caregivers, so this is certainly an area that should be explored more in future [65].
5Conclusions
Although ONS are sometimes prescribed due to poor oral health, studies have indicated that they may also increase the risk of oral disease, especially dental caries. Further study is needed into the dental disease potential of these supplements.
There is a need for increased communication and collaboration between nutritionists or medical professionals involved in the prescription of ONS and dental health professionals.
Recommendations
Local policies on ONS usage should include oral healthcare information and patients and/or their caregivers should be given preventative oral healthcare advice when oral nutritional supplements are prescribed
Where possible, screening for oral health issues should be incorporated into assessments of malnutrition risk and underlying causes of malnutrition.
Provision of oral health education for people prescribed ONS who are at risk of malnutrition/malnourished and/or their caregivers should include:
• Oral health techniques for prevention of caries and periodontal disease.
• Information regarding oral healthcare problems associated with high carbohydrate (sugar) containing foods and oral nutritional supplements.
• Recommend regular dental appointments for early diagnosis and treatment of any conditions.
Funding statement
This review is part of a larger study which is kindly supported by Cystic Fibrosis Ireland, the Health Research Board(HRB) and CiSA funding from University College Cork.
Conflict of interest
The authors declare that there is no conflict of interest.
Transparency declaration
The lead author affirms that this manuscript is an honest, accurate, and transparent account of the study being reported. The reporting of this work is compliant with PRISMA guidelines. The lead author affirms that no important aspects of the study have been omitted and that any discrepancies from the study as planned have been explained.
References
[1] | Cederholm T , Barazzoni R , Austin P , Ballmer P , Biolo G , Bischoff SC , et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clinical Nutrition. (2017) ;36: (1):49–64. |
[2] | EFSA Panel on Dietetic Products N, Allergies. Scientific and technical guidance on foods for special medical purposes in the context of Article 3 of Regulation (EU) No 609/2013. EFSA Journal. (2015) ;13: (11):4300. |
[3] | Kennelly S , Kennedy N , Flanagan Rughoobur G , Glennon Slattery C , Sugrue S . The use of oral nutritional supplements in an Irish community setting. Journal of Human Nutrition and Dietetics. (2009) ;22: (6):511–20. |
[4] | Care NCCfA. Nutrition support for adults: oral nutrition support, enteral tube feeding and parenteral nutrition. 2006. |
[5] | Weimann A , Braga M , Harsanyi L , Laviano A , Ljungqvist O , Soeters P , et al. ESPEN guidelines on enteral nutrition: surgery including organ transplantation. Clinical Nutrition. (2006) ;25: (2):224–44. |
[6] | Lochs H , Dejong C , Hammarqvist F , Hébuterne X , Leon-Sanz M , Schütz T , et al. ESPEN guidelines on enteral nutrition: gastroenterology. Clinical Nutrition. (2006) ;25: (2):260–74. |
[7] | Volkert D , Berner Y , Berry E , Cederholm T , Bertrand PC , Milne A , et al. ESPEN guidelines on enteral nutrition: geriatrics. Clinical Nutrition. (2006) ;25: (2):330–60. |
[8] | Anker S , John M , Pedersen P , Raguso C , Cicoira M , Dardai E , et al. ESPEN guidelines on enteral nutrition: cardiology and pulmonology. Clinical Nutrition. (2006) ;25: (2):311–8. |
[9] | Arends J , Bodoky G , Bozzetti F , Fearon K , Muscaritoli M , Selga G , et al. ESPEN guidelines on enteral nutrition: non-surgical oncology. Clinical Nutrition. (2006) ;25: (2):245–59. |
[10] | Gall M , Harmer J , Wanstall H . Prescribing of oral nutritional supplements in Primary Care: can guidelines supported by education improve prescribing practice? Clinical Nutrition. (2001) ;20: (6):511–5. |
[11] | Geraghty AA , McBean L , Browne S , Dominguez Castro P , Reynolds CME , Hanlon D , et al. Disparities in Oral Nutritional Supplement Usage and Dispensing Patterns across Primary Care in Ireland: ONSPres Project. Nutrients. (2022) ;14: (2):338. |
[12] | Streicher M , Themessl-Huber M , Schindler K , Sieber CC , Hiesmayr M , Volkert D . Who receives oral nutritional supplements in nursing homes? Results from the nutritionDay project. Clin Nutr. (2017) ;36: (5):1360–71. |
[13] | Li M , Zhao S , Wu S , Yang X , Feng H . Effectiveness of Oral Nutritional Supplements on Older People with Anorexia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients. (2021) ;13: (3). |
[14] | Milne AC , Potter J , Vivanti A , Avenell A . Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database of Systematic Reviews. 2009: (2). |
[15] | Milne AC , Avenell A , Potter J . Meta-Analysis: Protein and Energy Supplementation in Older People. Annals of Internal Medicine. (2006) ;144: (1):37–48. |
[16] | Stratton RJ , Elia M . Encouraging appropriate, evidence-based use of oral nutritional supplements. Proceedings of the Nutrition Society. (2010) ;69: (4):477–87. |
[17] | Cereda E , Pisati R , Rondanelli M , Caccialanza R . Whey Protein, Leucine- and Vitamin-D-Enriched Oral Nutritional Supplementation for the Treatment of Sarcopenia. Nutrients. (2022) ;14: (7):1524. |
[18] | Norman K , Pirlich M , Smoliner C , Kilbert A , Schulzke JD , Ockenga J , et al. Cost-effectiveness of a 3-month intervention with oral nutritional supplements in disease-related malnutrition: a randomised controlled pilot study. European Journal of Clinical Nutrition. (2011) ;65: (6):735–42. |
[19] | Simmons SF , Zhuo X , Keeler E . Cost-effectiveness of nutrition interventions in nursing home residents: A pilot intervention. The Journal of Nutrition, Health & Aging. (2010) ;14: (5):367–72. |
[20] | Coffey N , O’ Leary F , Burke F , Roberts A , Howlett C , Plant B , et al. Oral Nutritional Supplements: Sugar Content and Potential Dental Implications. Gerodontology.n/a(n/a). |
[21] | James SL , Abate D , Abate KH , Abay SM , Abbafati C , Abbasi N , et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. (2018) ;392: (10159):1789–858. |
[22] | Bukhari OM . Dental caries experience and oral health related quality of life in working adults. The Saudi Dental Journal. (2020) ;32: (8):382–9. |
[23] | Paśnik-Chwalik B , Konopka T . Impact of periodontitis on the Oral Health Impact Profile: A systematic review and meta-analysis. Dental and Medical Problems. (2020) ;57: (4):423–31. |
[24] | Baiju RM , Peter E , Varghese NO , Sivaram R . Oral Health and Quality of Life: Current Concepts. J Clin Diagn Res. (2017) ;11: (6):ZE21–ZE6. |
[25] | Tanaka T , Takahashi K , Hirano H , Kikutani T , Watanabe Y , Ohara Y , et al. Oral Frailty as a Risk Factor for Physical Frailty and Mortality in Community-Dwelling Elderly. J Gerontol A Biol Sci Med Sci. (2018) ;73: (12):1661–7. |
[26] | Peres MA , Macpherson LMD , Weyant RJ , Daly B , Venturelli R , Mathur MR , et al. Oral diseases: a global public health challenge. The Lancet. (2019) ;394: (10194):249–60. |
[27] | WHO. Guideline: sugars intake for adults and children. World Health Organization Geneva, Switzerland: WHO Press. 2015. |
[28] | Moynihan P . Sugars and Dental Caries: Evidence for Setting a Recommended Threshold for Intake. Adv Nutr. (2016) ;7: (1):149–56. |
[29] | McKenna G , Hayes M , DaMata C . Management of Caries in Older Adults. In: Hogue C-M, Ruiz JG, editors. Oral Health and Aging. Cham: Springer International Publishing; 2022. p. 131-44. |
[30] | Steele J , O’Sullivan I . Executive summary: adult dental health survey 2009. London: The Health and Social Care Information Centre. 2011. |
[31] | Dentino A , Lee S , Mailhot J , Hefti AF . Principles of periodontology. Periodontology. (2013) ;61: (1):16–53. |
[32] | Brown LJ , Loe H . Prevalence, extent, severity and progression of periodontal disease. Periodontology. (1993) ;2: (1):57–71. |
[33] | Laniado N , Levin L , Lamster I . Management of Periodontal Disease in Older Adults. In: Hogue C-M, Ruiz JG, editors. Oral Health and Aging. Cham: Springer International Publishing; 2022. p. 109-29. |
[34] | Reuland-Bosma W , van Dijk J . Periodontal disease in Down’s syndrome: a review. J Clin Periodontol. (1986) ;13: (1):64–73. |
[35] | Grossi SG , Zambon JJ , Ho AW , Koch G , Dunford RG , Machtei EE , et al. Assessment of risk for periodontal disease. I. Risk indicators for attachment loss. Journal of Periodontology. (1994) ;65: (3):260–7. |
[36] | Whelton H . Oral health of Irish adults 2000–2002: final report-April 2007/H. Whelton...[et al.]. -3962. 2007. |
[37] | Bostrom L , Linder LE , Bergstrom J . Influence of smoking on the outcome of periodontal surgery. A 5-year follow-up. J Clin Periodontol. (1998) ;25: (3):194–201. |
[38] | Bergstrom J , Bostrom L . Tobacco smoking and periodontal hemorrhagic responsiveness. J Clin Periodontol. (2001) ;28: (7):680–5. |
[39] | Lula EC , Ribeiro CC , Hugo FN , Alves CM , Silva AA . Added sugars and periodontal disease in young adults: an analysis of NHANES III data. Am J Clin Nutr. (2014) ;100: (4):1182–7. |
[40] | Figuero E , Nóbrega DF , García-Gargallo M , Tenuta LMA , Herrera D , Carvalho JC . Mechanical and chemical plaque control in the simultaneous management of gingivitis and caries: a systematic review. Journal of Clinical Periodontology. (2017) ;44: (S18):S116–S34. |
[41] | Chapple ILC , Bouchard P , Cagetti MG , Campus G , Carra M-C , Cocco F , et al. Interaction of lifestyle, behaviour or systemic diseases with dental caries and periodontal diseases: consensus report of group 2 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. Journal of Clinical Periodontology. (2017) ;44: (S18):S39–S51. |
[42] | Vila T , Sultan AS , Montelongo-Jauregui D , Jabra-Rizk MA . Oral Candidiasis: A Disease of Opportunity. J Fungi (Basel). (2020) ;6: (1). |
[43] | Akpan A , Morgan R . Oral candidiasis. Postgraduate Medical Journal. (2002) ;78: (922):455. |
[44] | Lewis MAO , Williams DW . Diagnosis and management of oral candidosis. British Dental Journal. (2017) ;223: (9):675–81. |
[45] | Farah CS , Lynch N , McCullough MJ . Oral fungal infections: an update for the general practitioner. Australian Dental Journal. (2010) ;55: (s1):48–54. |
[46] | Man A , Ciurea CN , Pasaroiu D , Savin A-I , Toma F , Sular F , et al. New perspectives on the nutritional factors influencing growth rate of Candida albicans in diabetics. An in vitro study. Mem Inst Oswaldo Cruz. (2017) ;112: (9):587–92. |
[47] | Liberati A , Altman DG , Tetzlaff J , Mulrow C , Gøtzsche PC , Ioannidis JPA , et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. (2009) ;339: :b2700. |
[48] | Bramer WM , Rethlefsen ML , Kleijnen J , Franco OH . Optimal database combinations for literature searches in systematic reviews: a prospective exploratory study. Systematic Reviews. (2017) ;6: (1):245. |
[49] | Sterne JA , Hernán MA , Reeves BC , Savović J , Berkman ND , Viswanathan M , et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. (2016) ;355: :i4919. |
[50] | Withers CA , Lewis MJ , Gosney MA , Methven L . Potential sources of mouth drying in beverages fortified with dairy proteins: A comparison of casein- and whey-rich ingredients. Journal of Dairy Science. (2014) ;97: (3):1233–47. |
[51] | Castro Ramiro J , Giacaman Rodrigo A , Castro RJ , Giacaman RA , Arthur Rodrigo A , Maltz M , et al. Cariogenicity of a Milk-Based Drink Used as a Dietary Supplement for Older Adults Using a Root Caries Experimental Model. Caries Research. (2019) ;53: (1):76–83. |
[52] | Jung C , Molinari N , Bouhlel A , Ruimy R , Prêcheur I . Thickened Drinks and Oral Nutritional Supplements as Potential Reservoirs of Oral Microorganisms: Microbial Assays In Vitro. Research in Gerontological Nursing. 2020. |
[53] | Stillhart A , Wegehaupt FJ , Nitschke I , Attin T , Srinivasan M . Cariogenic potential of oral nutritional supplements measured by intraoral plaque pH telemetry. Clinical Nutrition. (2021) ;40: (5):3448–53. |
[54] | Baena-Monroy T , Moreno-Maldonado V , Franco-Martínez F , Aldape-Barrios B , Quindós G , Sánchez-Vargas LO . Candida albicans, Staphylococcus aureus and Streptococcus mutans colonization in patients wearing dental prosthesis. Med Oral Patol Oral Cir Bucal. (2005) ;10 Suppl 1: :E27–39. |
[55] | Hopkinson JB . Nutritional support of the elderly cancer patient: The role of the nurse. Nutrition. (2015) ;31: (4):598–602. |
[56] | Jadczak AD , Visvanathan R . Anorexia of aging-an updated short review. The Journal of Nutrition, Health & Aging. (2019) ;23: (3):306–9. |
[57] | Jones S . Nutritional interventions for preventing malnutrition in people with dementia. Nursing Older People. (2020) ;32: (3). |
[58] | Tan MW , Yee SH , Kaufman LB . Nutrition, dietary modifications and palliative dental care in dementia patients: A case study. Journal of the American Geriatrics Society. (2019) ;67: :S271–S2. |
[59] | Precheur I , Chevalier M . The fight against malnutrition in older adults: new aproaches in dentistry. Gériatrie et Psychologie Neuropsychiatrie du Vieillissement. (2015) ;13: (1):22–30. |
[60] | Johnson LE , Dooley PA , Gleick JB . Oral nutritional supplement use in elderly nursing home patients. Journal of the American Geriatrics Society. (1993) ;41: (9):947–52. |
[61] | Touger-Decker R , Mobley C . Position of the Academy of Nutrition and Dietetics: oral health and nutrition. J Acad Nutr Diet. (2013) ;113: (5):693–701. |
[62] | Volkert D , Beck AM , Cederholm T , Cruz-Jentoft A , Goisser S , Hooper L , et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clinical Nutrition. (2019) ;38: (1):10–47. |
[63] | Moynihan P . Dietary therapy in chronically sick children: dental health considerations. Quintessence International. (2006) ;37: (6-10):444. |
[64] | More FG , Sasson LM , Godfrey EM , Sehl RB . Collaboration Between Dietetics and Dentistry: Dietetic Internship in Pediatric Dentistry. Top Clin Nutr. (2005) ;20: (3):259–68. |
[65] | Jepsen S , Blanco J , Buchalla W , Carvalho JC , Dietrich T , Dörfer C , et al. Prevention and control of dental caries and periodontal diseases at individual and population level: consensus report of group 3 of joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. Journal of Clinical Periodontology. (2017) ;44: (S18):S85–S93. |
Appendices
Appendix 1-Example of search strategy
Database used: Embase
No. Query Results
#11. #1 AND #6
#10. #1 AND #5
#9. #1 AND #4
#8. #1 AND #3
#7. #1 AND #2
#6. ‘periodont*’
#5. ‘candida’ OR ‘candidiasis’ OR ‘thrush’
#4. ‘dental caries’ OR ‘caries’ OR ‘tooth decay’
#3. ‘oral health’ OR ‘oral hygiene’
#2. dental
#1. ‘oral nutritional supplement’/exp OR ‘oral
nutritional supplement’ OR ‘ONS’
Appendices
Appendix 2: Nutritional Information of “Bebida Láctea Anos Dorados”
| 100g | 1 portion (25 g) | |
| Energy (kcal) | 400 | 100 |
| Protein (g) | 18.0 | 4.5 |
| Total Fat (g) | 10.0 | 2.5 |
| Total Carbohydrate (g) | 59.5 | 14.8 |
| Lactose (g) | 28.0 | 7.0 |
| Sucrose and other simple sugars (g) | 8.0 | 2.0 |
| Fibre (g) | 1.0 | 0.3 |



