Linking Social Cognition, Parvalbumin Interneurons, and Oxytocin in Alzheimer’s Disease: An Update
Abstract
Finding a cure for Alzheimer’s disease (AD) has been notoriously challenging for many decades. Therefore, the current focus is mainly on prevention, timely intervention, and slowing the progression in the earliest stages. A better understanding of underlying mechanisms at the beginning of the disease could aid in early diagnosis and intervention, including alleviating symptoms or slowing down the disease progression. Changes in social cognition and progressive parvalbumin (PV) interneuron dysfunction are among the earliest observable effects of AD. Various AD rodent models mimic these early alterations, but only a narrow field of study has considered their mutual relationship. In this review, we discuss current knowledge about PV interneuron dysfunction in AD and emphasize their importance in social cognition and memory. Next, we propose oxytocin (OT) as a potent modulator of PV interneurons and as a promising treatment for managing some of the early symptoms. We further discuss the supporting evidence on its beneficial effects on AD-related pathology. Clinical trials have employed the use of OT in various neuropsychiatric diseases with promising results, but little is known about its prospective impacts on AD. On the other hand, the modulatory effects of OT in specific structures and local circuits need to be clarified in future studies. This review highlights the connection between PV interneurons and social cognition impairment in the early stages of AD and considers OT as a promising therapeutic agent for addressing these early deficits.
INTRODUCTION
Alzheimer’s disease (AD) is a neurodegenerative disease with an enormous socioeconomic burden. Almost 60 million people worldwide suffer from this disease, and the numbers are expected to triple by 2050 [1]. The main histopathological hallmarks of AD include the extracellular deposits of insoluble amyloid-β protein (amyloid-β plaques) and intracellular aggregates of hyperphosphorylated tau protein, accompanied by neuronal loss and gradual atrophy of the brain [2]. Progressive memory loss is often referred to as the most prominent symptom. However, besides the gradually degrading episodic memory, patients diagnosed with AD often experience affective and depressive symptoms, including mood swings, apathy, anxiety, and social withdrawal [3–5].
In this review, we focus on deficits in social cognition in early AD and relate them to the progressive decline of parvalbumin (PV) interneurons, also referred to as fast-spiking interneurons. To this date, few studies have addressed the relationship between PV interneurons and deficits in the social cognitive domain. We focus the review on rodent AD models, which form an indispensable part of current research. In the last section, we discuss oxytocin (OT) as a potent modulator of both PV interneurons and social memory, with additional beneficial effects against AD-related pathology.
Early stage of AD and social behavior
Temporal lobes have long been considered the first morphologically and functionally affected areas, primarily the entorhinal cortex, amygdala [6–8], and the hippocampus [9–10]. These are the first structures affected by the extracellular amyloid-β plaque accumulation, the main pathological hallmark of AD [11]. Moreover, their volume is already significantly reduced with the onset of the first cognitive symptoms [8]. Besides their roles in cognition and memory, they are essential for emotional processing and social behaviors [12, 13].
According to Jost and Grossberg, social withdrawal is one of the earliest noticeable symptoms, occurring up to almost three years before the diagnosis in 40% of AD patients [3]. In addition, recent cohort studies confirmed that social isolation, such as living alone or not participating in social activities, dramatically increases the risk of developing dementia and even speeds its progression [14–16]. This forms a vicious cycle of social behavior dysfunction with the disease. Dissociating early symptoms from the causes of the disease yet remains a question of future research.
Animal models and early AD
Most current animal AD models are based on inserting one or more gene mutations associated with an early-onset form of AD into the rodent genome. These include mutations in amyloid-β precursor protein (APP) in hAPPJ20 [17], APPNL - F [18], and Tg2576 mice [19], or combined APP and presenilin mutations in 5xFAD [20] and APP/PS1 mice [21] and TgF344-AD rats [22]. Such insertions in these models generally lead to progressive amyloidosis and age-dependent cognitive decline [18–22]. Models involving tauopathies do exist, but amyloid models vastly outnumber them. In any case, AD is a multifactorial disease, and it is impossible to completely mimic the entire pathology in rodents, regardless of the model used. It is necessary to carefully interpret results from animal studies, as they are mostly limited to modeling early-onset familial AD, which involves only 5–6% of dementias [23]. The research also comprises various types of animal models, strains, and animals themselves, including different ages when undergoing the experiments. Despite comparable pathological features, the overall manifestation and progression of the disease can differ. Nevertheless, many pathological and behavioral alterations overlap in these AD models, including those in social behavior.
Similar to human patients with AD, rodent models also often exhibit early deficits in the social domain, preceding dysfunction in spatial memory. Reduced sociability and social recognition were reported in various AD models, starting as soon as three months [24–28] in both mice and rats. A study directly comparing social and spatial behavior found that 6-month-old APP and APP/PS1 female mice showed reduced sociability but no impairment in spontaneous spatial alternation [29]. Similar social deficits were observed in another study, where the APP/PS1 mice of the same age were less interested in social encounters per se but the authors also reported a hyperactivity in tests for general exploratory activity [30]. Progressive worsening of spatial learning and reference memory then first appears around five to six months [31–34], although some authors reported memory deficits starting even later— around nine to ten months of age in mice [19, 35, 36] and seven to eight months in rats [37]. Nevertheless, considerable variability is observed when probing deficits related to spatial learning. This variability seems to be influenced by a number of factors, namely various experimental design, evaluated parameters or specific genetic background of AD models used. Altogether, early differences in the social domain across various rodent models appear to be more consistent.
When addressing the impact of social isolation on AD progression in mice, it was shown that three-month long isolation from their conspecifics significantly increases plaque deposits in the hippocampus and worsens memory performance [38–40]. Apparently, there is a strong interconnection between the lack of social interactions and the progression of the disease in both human patients and animal models. The early symptoms of genetic animal models more or less resemble that of humans. Yet, deviations related to emotional states and social cognition have not received adequate attention in research, although examining them in the context of the ongoing histopathological changes could significantly contribute to early intervention.
PV interneurons and E/I balance in AD
Numerous brain pathologies are characterized by an imbalance between excitatory and inhibitory actions in brain microcircuits, and AD is no exception. The excitatory/inhibitory (E/I) balance refers to the constant ratio of excitatory and inhibitory currents on individual pyramidal neurons [41]. Maintaining this balance in homeostasis is critical for efficient neural coding in the brain [42]. The equilibrium, however, undergoes dynamic modulation through short-term plasticity, influencing the strength and timing of individual synaptic inputs and modulating the signal outputs in the hippocampal circuits [43, 44]. A precisely controlled E/I balance is also essential for the formation and control of neuronal oscillations [45, 46]. E/I balance is primarily maintained by PV interneurons that modulate and synchronize pyramidal neurons [47–49]. In the hippocampus, these interneurons contribute to the generation of theta (4–10 Hz) [48] and gamma (30–120 Hz) [49] oscillations, along with sharp-wave ripples (150–200 Hz) [50]. These oscillations are crucial for long-term memory formation and information processing [51, 52].
Dysfunction of PV interneurons in the hippocampus inevitably leads to alterations in theta and gamma oscillations, which in turn may disrupt spatial working and reference memory [53]. Furthermore, optogenetic inhibition of PV interneurons also inhibits sharp wave-ripples [50], which are crucial for consolidating hippocampus-dependent memories [54]. The loss of PV interneurons or the loss of their proper functioning is accompanied by network disturbance and cognitive dysfunction in various psychiatric diseases [55]. Although the AD pathology was studied primarily in terms of dysfunction of glutamatergic and cholinergic systems, there is growing evidence that GABAergic, i.e., inhibitory signaling is affected in the early stages of this disorder, leaving a negative impact on the E/I balance [27, 55–60].
Disrupted E/I balance or altered function of PV interneurons was reported in various AD models [61–64]. Furthermore, reduced inhibition is linked to hypersynchronous or epileptiform activity, which was observed in these models as well [65–68]. In fact, altered excitability of PV interneurons in AD occur long before memory deficits appear, especially in the hippocampus [69–70]. Some authors suggest that aberrations in PV interneuron functioning might precede the increasing amyloid-β plaque accumulation [71–72], which starts at approximately four months in APP/PS1 mice [73] and six months in APP/PS1 rats [22]. On the other hand, the presence of amyloid-β might further aggravate the disrupted GABAergic activity and contribute to reduced dendritic inhibition of pyramidal cells in the hippocampus [74]. Findings confirming the early disruption of PV interneurons in AD contribute to emerging efforts to map their role in social cognition.
PV interneurons and memory
PV interneurons play an important role in various types of memory, including spatial, fear, and social memory. In the hippocampal area CA1, which is well known for processing spatial information, these interneurons are crucial for spatial working memory but do not seem to be involved in spatial reference memory [75, 76]. In addition, mice with NMDA receptor ablation on PV interneurons demonstrated impaired spatial working memory and both short- and long-term object recognition memory, but intact spatial reference learning [53]. Despite some debate regarding the role of PV interneurons in phase-dependent recurrent inhibition in the medial entorhinal cortex [77], it has been shown that PV interneurons are necessary for maintaining the grid cells’ activity in this area, which is crucial for spatial representation [78]. PV interneurons were also studied in the context of memory consolidation. Specifically, they were shown to be critical for fear memory consolidation. They serve as the cellular substrate for preserving fear memories and are essential for associated high-frequency oscillatory activity in the hippocampus [79]. These interneurons also promote network plasticity in hippocampal CA1 and, consequently, long-term fear memory formation [51]. Furthermore, successful fear memory consolidation relies on coherent communication between the hippocampus and the neocortex, which is mediated by PV interneurons [80].
Recently, it was shown that PV interneurons might be a core of social memory in various brain areas, specifically the hippocampal dorsal CA2 and its connected ventral CA1 regions [81–84]. The dorsal CA2 is enriched in PV interneurons compared to other hippocampal areas [85]. This area is critical for the encoding, consolidation, and recall processes related to social memories [81]. The “social novelty signal” arises from the activity of the supramammillary nucleus of the hypothalamus, which sends its projections to the CA2 [86, 87]. This pathway provides inhibitory tone by stimulating PV basket interneurons and synchronizes the firing of pyramidal neurons [87]. Contrary to dorsal CA2, ventral CA1 only stores social memory engrams [82]. Higher calcium activity of PV interneurons in ventral CA1 was observed when approaching novel mice, while hyperexcitability or inactivation of these interneurons impaired social discrimination [83]. Interfering with the activity of these interneurons in any direction thus affects the ability to identify familiar and stranger conspecifics.
Long-term depression is a type of plasticity unique to the hippocampal CA2 region [88]. This type of plasticity depends on maturation of PV interneurons during adolescence, mainly due to receptor tyrosine-protein kinase ErbB4 signaling and the formation of perineuronal nets around PV interneurons [84]. Inhibitory plasticity appears to be the core mechanism for social memory formation [84]. Indeed, Rey and colleagues confirmed this finding by showing that the disrupted function of PV interneurons and loss of perineuronal nets in CA2 led to social memory deficits in Tg2576 mice, a double mutant AD model [27]. Moreover, the authors reported lower numbers of PV interneurons and lower intensities of fluorescent anti-PV staining in these cells [27]. Interestingly, dysfunction of PV interneurons was not manifested in deficits in spatial memory, confirming the exclusivity of CA2 in social memory processing [27]. On the other hand, chronic but not acute silencing of PV interneurons in ventral CA1 affected the recognition of novel objects [83]. Thus, chronic manipulation of PV interneurons in ventral CA1 may impact both social and non-social aspects of memory.
Various neuromodulators are involved in social coding. Activation of delta-opioid and cannabinoid type 1 receptors induces long-term depression of inhibitory transmission in the CA2, which is required for social memory formation [88, 89]. Furthermore, oxytocinergic signalization modulates both excitatory pyramidal cells and PV interneurons in the CA2 [90]. Activation of hippocampal OT receptors (OTRs) is critical for discriminating between social memories [91] and maintaining long-term social recognition memory [92].
As mentioned previously, prolonged social isolation of either young or old mice with AD-related genes can exacerbate the progression of the disease [38–40]. Deng et al. reported that social isolation also reduces the PV interneuron number in the ventral hippocampus [83]. Thus, social isolation could be another factor that negatively affects interneurons, making them vulnerable to pathological events. Altogether, the activity of PV interneurons is not only crucial for social memory but also plays significant roles in various aspects of spatial and declarative memory processes. Gradual impairment of these interneurons will undoubtedly manifest across many cognitive domains.
PV interneuron dysfunction in the early stage
In the earliest stages of AD, when the first non-cognitive deficits appear, neuronal networks seem hyperactive [93–95]. This hyperactive state can cause epileptiform activity, as observed in patients with AD [96] and animal models [65, 95]. It was reported that transgenic hAPPJ20 mice have decreased levels of a voltage-gated sodium channel subunit (Nav1.1) on PV interneurons, leading to reduced inhibitory synaptic activity [65]. The dysfunction of PV interneurons likely contributes to epileptiform discharges in these mice [65]. Improper inhibition is possibly one of the main factors in neuronal hyperexcitability in AD [69]. In the following sections, we discuss other PV interneuron abnormalities that have been reported in relation to AD. Next, we outline selective PV interneuron manipulation as a potential mechanism to restore the proper functioning of neuronal networks in AD.
Morphological changes. PV interneurons exhibit highly plastic properties, and early in the disease, they may compensate for pathological changes and keep the E/I balance. To do so, their morphological and functional features change. In a mouse model with APP mutation, it was shown that the PV interneurons are resilient to amyloid-β plaques and can compensate morphologically for the amyloid-β-induced hyperactivity of pyramidal cells [97]. While the density of PV cells remains unchanged, the number of their terminals near plaques increases, and the synaptic area around initial segments significantly enlarges [97]. The increased dendrite length of PV interneurons and greater dendritic complexity were also described in 9- to 12-month-old APP/PS1 rats [98]. Similarly, enhanced synaptic projections of PV interneurons were observed in the hippocampus of 3-month-old APP/PS1 mice [61]. Together with morphological changes in the PV interneurons, higher levels of PV protein and GABAergic neuron marker (GAD67) were detectable in studied rats and mice, respectively [61, 98].
Functional changes. Functional changes may accompany morphological adaptations. Recently, it was proposed that the aberrant activity of PV interneurons changes throughout the disease, starting with hyperactive interneurons [63]. The authors showed the initial hyperexcitability of PV interneurons and gradual pathological changes in 4-month-old mice lead to aberrant synaptic transmission— reduction in excitatory input and inhibitory output— and their hypoactivation later in life, at about six months of age. Increased GABAergic inhibition might, in fact, act as a compensatory mechanism for overexciting pyramidal cells, which is caused by surrounding amyloid-β plaques [94, 95]. PV interneurons are more resilient to AD pathology [98, 99], and their activity is unaffected by the proximity of amyloid-β plaques [100].
Despite initial compensatory mechanisms, the PV interneurons are highly vulnerable to metabolic and oxidative stress during aging [101]. Further progression of AD takes its toll, and the PV interneurons start to malfunction, as indicated in the study of Hijazi et al. [63]. Decreased inhibitory transmission may then be the culprit in disrupted E/I balance and contribute to the progression of the disease, including brain hyperexcitability [70]. Indeed, decreased activity of PV interneurons was described in 8- to 11-month-old APP/PS1 mice [100]. Here, it is important to note that some interneurons might be affected by AD pathology even sooner than PV interneurons, for instance, somatostatin interneurons [102]. According to AD pathology, they increase their activity with proximity to the amyloid-β plaques and can cause or contribute to further hypoactivity of PV interneurons [94, 100]. However, their overall role in early AD and social cognition remains uncertain.
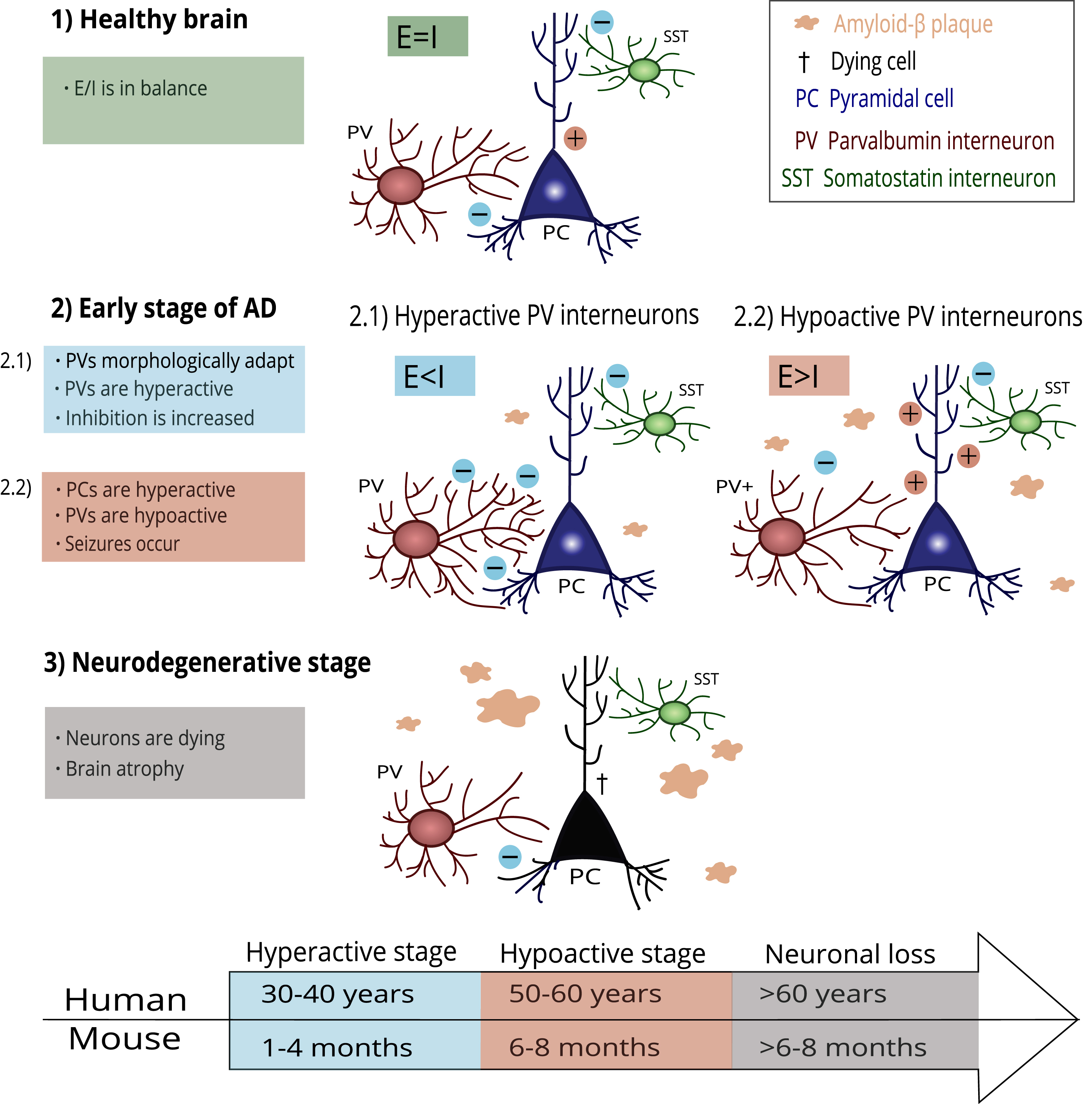
The impaired function of PV interneurons in hippocampal CA2 is accompanied by reduced inhibitory control in CA2, resulting in hyperexcitability of the pyramidal neurons and social memory deficits in Tg2576 mice [27]. This finding follows the general view that poor inhibitory transmission results in hyperexcitability of pyramidal cells, further leading to neurotoxicity and neuronal loss [103]. The PV interneuron damage progression in AD is illustrated in Fig. 1.
Fig. 1
A possible mechanism of PV interneuron damage progression in AD. Healthy brains keep the E/I balance at a steady state (1). In the early stage of AD, the first amyloid-β plaques occur. PV interneurons adapt to this process through morphological and functional changes (2.1). PV interneurons become hyperactive, and the E/I balance is disrupted due to increased inhibition. However, PV interneurons cannot compensate for the damage and start hypofunction (2.2). Pyramidal cells increase firing due to decreased inhibition, and epileptiform seizures appear. In the final stage of AD, progressing pathological processes lead to irreversible damage and neuronal loss. The timeline under the scheme shows an illustrative comparison of AD-related histopathological changes in humans and mice according to their lifespan.
In contrast to previously reported hyperactivity in the hippocampus at approximately four months of age [63], other studies showed either hypoactivity or a reduced number of PV interneurons earlier in life. Reduced excitability of PV interneurons was observed in hAPPJ20 mice [72], similar to decreasing numbers in TgCRND8 mice [104] in already 1-month-old individuals. In addition, hypoactivity of PV interneurons was reported in the prefrontal cortex of APP/PS1 mice as early as nine to eleven weeks [105]. This inconsistency might arise from different animal models used and brain structures being studied, as summarized in Table 1. The variability in results could be also attributed to the particular research methods selected, e.g., ex vivo recording, which clearly differs from freely moving animals.
Table 1
Reported differences in PV interneuron morphology, excitability, and overall numbers
| Study | Strain | Age | PV interneurons | Structure |
| Mahar et al. (2017) [104] | TgCRND8 mice | 1 m | ↓ number | hippocampus |
| Mondragón-Rodríguez et al. (2018) [72] | hAPPJ20 mice | 1 m | ↓ excitability | subiculum |
| Shu et al. (2023) [105] | APP/PS1 mice | 2-3 m | ↓ activity | prefrontal cortex |
| ↓ number | ||||
| Hollnagel et al. (2019) [61] | APP/PS1 mice | 3 m | ↑ density of projections | hippocampus |
| ↑ PV immunoreactivity | ||||
| Hijazi et al. (2020) [63] | APP/PS1 mice | 4 m | ↑ excitability | hippocampus |
| 6 m | ↑ PV immunoreactivity | |||
| no difference in number | ||||
| ↓ activity | ||||
| ↓ PV immunoreactivity | ||||
| no difference in number | ||||
| Algamal et al. (2022) [100] | APP/PS1 mice | 8–11 m | ↓ activity | somatosensory cortex |
| Verret et al. (2012) [65] | hAPPJ20 mice | 4–7 m | ↓ Nav1.1 subunit levels | parietal cortex |
| ↓ amplitude of AP | ||||
| Li et al. (2022) [107] | 5xFAD mice | 5-6 m | ↓ PV levels | hippocampus |
| ↓ number | ||||
| Ali et al. (2020) [103] | 5xFAD mice | 6–9 m | ↓ number | frontal cortex |
| Rey et al. (2022) [27] | Tg2576 mice | 9 m | ↓ number | hippocampus |
| Giesers & Wirths (2020) [108] | 5xFAD mice | 12 m | ↓ number | hippocampus |
| Tg4-42 mice | 12 m | no difference in number | ||
| Mackenzie-Gray Scott et al. | 5xFAD mice | 3–6 m | no difference in number | hippocampus |
| (2022) [99] | 12+ m | ↓ number | ||
| Takahashi et al. (2020) [111] | APP/PS1 mice | 10 m | ↓ number | hippocampus |
| Sun et al. (2021) [110] | TgF344-AD rats | 9 m | ↓ PV levels | hippocampus |
| Morrone et al. (2022) [98] | TgF344-AD rats | 9–12 m | ↑ complexity and/or length of dendrites | hippocampus |
| 15 m | ↓ complexity and/or length of dendrites | |||
| Sos et al. (2020) [97] | APPNL - F mice | 18 m | ↑ enlarged synapses | hippocampus |
| no difference in density | ||||
| Zallo et al. (2018) [109] | 3xTg-AD mice | 18 m | ↓ number | hippocampus |
It should be pointed out that PV interneuron dysfunction might be place- and cell-type specific, which is supported by the observation that the basket cells, but not the bistratified or axo-axonic cells, had reduced activity during memory consolidation in the hippocampal CA1 region of 3-month-old 5xFAD mice [62]. To our knowledge, no study has addressed the impairment of specific cell types in dorsal CA2, where social memories form.
PV interneurons in the neurodegenerative stage
Studies across human and animal research have reported a decline in PV interneuron numbers. Immunohistochemical analysis of the brains of 70–80 years old AD patients showed over 60% loss of PV interneurons in some hippocampal subfields (dentate gyrus, CA1, and CA2), compared to healthy brains [106]. Similarly, loss of PV or PV interneurons was described in the hippocampus of various AD models with amyloid-β plaque accumulation [107–110]. Takahashi et al. reported a 50% reduction of PV interneurons in the hippocampus of 10-month-old APP/PS1 mice [111], similar to the loss of pyramidal cells reported in APPSLPS1KI mice of the same age [112]. Most research studies use anti-PV antibodies as a marker for counting PV interneurons. However, the decrease in staining when using anti-PV antibodies does not necessarily account for neuronal loss; decreased number of PV interneurons can also be explained by decreased PV expression in the neuronal cells [113]. It is crucial to differentiate between these two phenomena as they may have opposing effects. Some studies suggest that PV cell loss leads to decreased inhibition, whereas loss of PV in cells slows the decay of Ca2 + kinetics and enhances synaptic facilitation, which in turn favors inhibition [113–116]. It is, therefore, questionable if the observed decrease in PV interneurons reflects the actual neuronal loss. The number of PV interneurons was reported to be reduced as early as in 1-month-old TgCRND8 mice [104] and 2-to 3-month-old APP/PS1 mice [105]. Thus, the early PV reduction delineated in these studies might reflect early deficits in PV expression rather than dying PV interneurons.
Manipulating the PV interneuron activity
One advantage of animal models is the possibility of interrogating the function of interneurons and seeing their role in AD-related behavior. For example, enhancing GABAergic activity directly by GABA administration was already shown to improve cognitive performance in 2-month-old APP/PS1 mice [117]. Targeted manipulations of selected cells or brain circuits with the potential to modulate the E/I balance may further help to expand the knowledge about altered neuronal functioning or alleviate cognitive decline. Hijazi et al. showed that chemogenetic inhibition of PV interneurons earlier or activation later in life led to similar improvements in cognitive functioning and proposed opposite interventions according to the specific stage of the disease [63]. Furthermore, chronic optogenetic stimulation (40 Hz) of PV interneurons in the medial septal region of mice with double APP mutation restored the impaired slow gamma (30–60 Hz) oscillations in the hippocampus and supported memory retrieval [64]. Non-invasive 40 Hz visual stimulation was as efficient as the modulation of hippocampal activity and improved spatial recognition memory of the 5xFAD model of AD [118]. Such visual stimulation also lowered amyloid-β levels in the visual cortex [119]. It would be interesting to test how such stimulation affects social behaviors or recognition in early AD animal models. In another approach, we can target social modulators to alleviate early symptoms in the social domain. To achieve this, we consider OT a promising drug and discuss its benefits in the following section.
OXYTOCIN AND SOCIAL COGNITION
OT is a neuropeptide involved in many aspects of social behaviors, including empathy or social recognition [120–122]. Here, we will put oxytocinergic signalization in the context of social memory processing and focus on their modulatory outcomes in PV interneurons. As social memory is crucially dependent on dorsal hippocampal CA2 [91, 123, 124], we will highlight the modulation of this part.
Modulatory effects of OT in the hippocampus were already comprehensively described in another review [125]. In summary, the main oxytocinergic pathway leading to the hippocampus originates in the paraventricular nucleus of the hypothalamus [126]. OT acts on neurons at both presynaptic and postsynaptic levels [127], exerting widespread effects via G-protein coupled receptors [128]. Most notably, OT enhances the long-term potentiation in the hippocampal CA1 by activating protein kinase Mζ [129]. These processes are essential for maintaining long-term memories [130].
The hippocampal CA2/CA3 regions in mice are highly enriched in OTRs, and about 90% of hippocampal OTRs are expressed on excitatory pyramidal cells [91, 123]. Despite the relatively low presence of OTRs in GABAergic interneurons, about 50% of PV interneurons in the dorsal CA2/CA3 regions express OTRs [90, 91]. The high abundance of OTRs in CA2 led scientists to an assumption that these receptors might play a role in social memory. Indeed, deleting these receptors in the hippocampus led to impairments in discrimination between a novel and familiar mouse [91] and negatively affected the long-term storage of social memories, without affecting sociability per se [92, 131]. Moreover, oxytocinergic signalization is necessary for long-term social memory formation and persistence [131]. It is important to note that OT shapes neural activity in the hippocampus and other brain areas related to social behavior and memory, especially the amygdala and prefrontal cortex [132].
In general, OT increases the excitability of GABAergic interneurons, favoring inhibition [133]. This interneuronal depolarization is cell-specific and restricted to PV interneurons [134]. Therefore, OT indirectly inhibits the activity of pyramidal cells in hippocampal CA1 [135]. OTRs can similarly be found in the ventral part of CA1 [91]; however, to this date, no study has probed the effects of OT administration in this area on social recognition. Using a selective OTR agonist TGOT in the hippocampal CA2, it was found that PV interneurons and excitatory pyramidal cells are activated together [90]. The authors proposed that co-activation is necessary for shaping the burst-firing of pyramidal cells, causing a rapid and precise sequence of action potentials and modulating synaptic plasticity [90, 134]. Nevertheless, the exact mechanisms of the E/I balance modulation induced by OT in complex neural networks lie upon further investigation. Considering the profound effect on PV interneurons, the OT administration may be beneficial for improving some aspects of social cognition, including memory. This modulation might have more significant effects at certain stages of the disease, e.g., when the interneurons are hypoactive.
Oxytocin as a potent future modulator in AD
The therapeutic effects of OT have already been probed in other neuropsychiatric disorders, such as anxiety disorders, depression, and schizophrenia [136]. While autism is characterized by disruption in oxytocinergic signalization [137], AD patients seem to have unaltered OT plasma concentrations [138] and the number of OT-expressing neurons [139]. Therefore, administering OT in AD could function as a positive modulator of brain functions rather than a correction for impaired signaling. According to the ClinicalTrials.gov database, one clinical testing of intranasal OT administration for frontotemporal dementia is underway (NCT03260920), with three others done in the past (NCT01386333, NCT01937013, NCT01002300). In contrast, the effects of OT have not been tested yet in patients with AD and its influence on social memory is still limited to research on rodent AD models.
OT was found to improve social memory after a 2-h re-exposure interval when injected into the septal nuclei of rats [140]. Later studies revealed that OT in the hippocampus and medial amygdala has a positive impact on social memory [91, 141]. The OT levels rise significantly in the amygdala and hippocampus in mice 30 min after intranasal administration, proving its effects on the brain [142].
An increasing body of evidence suggests the beneficial effects of OT in the AD-affected brain. Recent findings reveal that OT can improve long-term potentiation in the hippocampus that is impaired due to the amyloid-β plaques, thereby facilitating the transmission of information between synapses [143]. In a further study, the authors injected OT in the ventricles or administered an OT derivative intranasally in mice with amyloid-β peptide-induced amnesia and observed memory improvements in spatial domains [144]. Neuroprotective effects of OT were described when using an aluminum-induced AD model in female rats [145]. The authors stated that OT administered daily for eight weeks suppressed pathological pathways associated with amyloid-β accumulation, reduced tau levels, acetylcholinesterase activity, and decreased levels of kinases promoting neuronal death, such as ERK1/2. Another study confirmed in APP/PS1 mice that a 6-week prolonged intranasal administration of OT reduced amyloid-β plaque size in the hippocampi and promoted the deposition in dense core plaques, which is thought to be a neuroprotective mechanism [146]. OT also has anti-inflammatory properties and can attenuate microglial overactivation, which is typical for the disease [146]. Moreover, OT administration rescued the social memory deficits in these mice [146]. Activation of OTRs also promotes hippocampal neurogenesis [147], which further augments the potential positive effects of OT treatment.
As outlined in the previous chapter, the precise mechanisms of OT on E/I balance are yet to be fully elucidated. Since OT promotes the activity of PV interneurons, its administration might strengthen the inhibitory tone and enhance burst firing, which could facilitate synaptic transmission between hippocampal CA2 and CA1 regions, e.g., during consolidation of social memories [90]. In addition, enhanced inhibitory activity might reduce epileptiform activity of neuronal networks [65]. The OT effects on the synapse and possible mitigation of some aspects of AD pathology are illustrated in Fig. 2.
Fig. 2
A schematic illustration of oxytocin effects on synapse and its potential therapeutic effects in AD brains. Oxytocin induces long-term potentiation by increasing levels of protein kinase Mζ, which regulates transcription of the AMPA receptors and their incorporation into the membrane [129, 148]. Oxytocin treatment may help to rescue the E/I imbalance in AD-affected brains, synchronize the firing of pyramidal cells by modulating the activity of PV interneurons, reduce microglial overactivation and support neuroprotective mechanisms.
![A schematic illustration of oxytocin effects on synapse and its potential therapeutic effects in AD brains. Oxytocin induces long-term potentiation by increasing levels of protein kinase Mζ, which regulates transcription of the AMPA receptors and their incorporation into the membrane [129, 148]. Oxytocin treatment may help to rescue the E/I imbalance in AD-affected brains, synchronize the firing of pyramidal cells by modulating the activity of PV interneurons, reduce microglial overactivation and support neuroprotective mechanisms.](https://content.iospress.com:443/media/jad/2023/96-3/jad-96-3-jad230333/jad-96-jad230333-g002.jpg)
Considering some limitations and discrepancies regarding oxytocinergic signalization in humans is essential. Rodent neural structures, like the hippocampus, may differ across species. For example, guinea pigs lack OTRs in the hippocampus [149]. The incomplete understanding of oxytocinergic signaling in humans poses potential challenges in translational research. However, it also presents an entirely new avenue for investigation that awaits exploration.
CONCLUDING REMARKS
Abnormal activity in GABAergic systems and observed altered E/I balance in humans with AD and its animal models show apparent involvement of PV interneurons. A growing interest has emerged in investigating their role during the early stages, preceding the full manifestation of the disease. Particular attention has been directed toward early changes in social cognition and social memory, aiming to establish a connection between early deficits and the aberrant activity of the PV interneurons. The relation between PV interneurons activity and social behavior in AD might be found more complex if proven true that PV interneurons transit from a hyperactive to a hypoactive state during the progression of the disease.
OT is likely a potent modulator of PV interneurons and social cognition, suggesting a prospective role in treating cognitive and social deficits. Although some clinical trials already included intranasal OT administration to alleviate some neuropsychiatric conditions, the focus on therapeutic effects in AD is still minor. Moreover, precise mechanisms of action and modulatory effects of OT need to be clarified in future studies, with the inevitable role of animal models. It would be interesting to test the OT’s local effects in socially engaged brain structures, such as the hippocampus, amygdala, or prefrontal cortex, and distinguish its modulatory role in specific cell types. Alternatively, the effects of OT may vary based on its administration at different phases, for example, during encoding, consolidation, or recall of social memories.
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
FUNDING
The writing of this review paper was supported by the Czech Science Foundation (GACR) grant 21-16667K and project No. LX22NPO5107 (MŠMT): funded by the European Union - Next Generation EU. Daniela Černotová and Karolína Hrůzová acknowledge support by the project Grant Schemes at Charles University, reg. no. CZ.02.2.69/0.0/0.0/19_073/0016935.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
REFERENCES
[1] | Nichols E , Steinmetz JD , Vollset SE , Fukutaki K , Chalek J , Abd-Allah F , Abdoli A , Abualhasan A , Abu-Gharbieh E , Akram TT , et al. ((2022) ) Estimation of the global prevalence of dementia in 2019 andforecasted prevalence in 2050: An analysis for the Global Burden ofDisease Study 2019. Lancet Public Health 7: , e105–e125. |
[2] | Bloom GS ((2014) ) Amyloid-β and tau: The trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol 71: , 505–508. |
[3] | Jost BC , Grossberg GT ((1996) ) The evolution of psychiatric symptoms in Alzheimer’s disease: A natural history study. J Am Geriatr Soc 44: , 1078–1081. |
[4] | Berger AK , Fratiglioni L , Forsell Y , Winblad B , Bäckman L ((1999) ) The occurrence of depressive symptoms in the preclinical phase ofAD. Neurology 53: , 1998–1998. |
[5] | Ismail Z , Gatchel J , Bateman DR , Barcelos-Ferreira R , Chantillon M , Jaeger J , Donovan NJ , Mortby ME ((2018) ) Affective and emotional dysregulation as pre-dementia risk markers: Exploring the mild behavioral impairment symptoms of depression, anxiety, irritability, and euphoria. Int Psychogeriatr 30: , 185–196. |
[6] | Poulin SP , Dautoff R , Morris JC , Barrett LF , Dickerson BC ((2011) ) Amygdala atrophy is prominent in early Alzheimer’s disease and relates to symptom severity. Psychiatry Res 194: , 7. |
[7] | Planche V , Manjon JV , Mansencal B , Lanuza E , Tourdias T , Catheline G , Coupe P ((2022) ) Structural progression of Alzheimer’s disease over decades: The MRI staging scheme. Brain Commun 4: , fcac109. |
[8] | Basso M , Yang J , Warren L , MacAvoy MG , Varma P , Bronen RA , van Dyck CH ((2006) ) Volumetry of amygdala and hippocampus and memory performance in Alzheimer’s disease. Psychiatry Res 146: , 251–261. |
[9] | Braak H , Braak E ((1991) ) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82: , 239–259. |
[10] | Rao YL , Ganaraja B , Murlimanju BV , Joy T , Krishnamurthy A , Agrawal A ((2022) ) Hippocampus and its involvement in Alzheimer’s disease: A review. 3 Biotech 12: , 55. |
[11] | Sepulcre J , Sabuncu MR , Becker A , Sperling R , Johnson KA ((2013) ) Invivo characterization of the early states of the amyloid-betanetwork. Brain 136: , 2239–2252. |
[12] | Bannerman DM , Grubb M , Deacon RMJ , Yee BK , Feldon J , Rawlins JNP ((2003) ) Ventral hippocampal lesions affect anxiety but not spatial learning. Behav Brain Res 139: , 197–213. |
[13] | Felix-Ortiz AC , Tye KM ((2014) ) Amygdala inputs to the ventral hippocampus bidirectionally modulate social behavior. J Neurosci 34: , 586–595. |
[14] | Huang AR , Roth DL , Cidav TM , Chung ScM S-E , Amjad H , Thorpe RJ Jr , Boyd CM , Cudjoe MTK ((2023) ) Social isolation and 9-year dementia risk in community-dwelling Medicare beneficiaries in the United States. J Am Geriatr Soc 71: , 765–773. |
[15] | Yin J , Lassale C , Steptoe A , Cadar D ((2019) ) Exploring the bidirectional associations between loneliness and cognitive functioning over 10 years: The English longitudinal study of ageing. Int J Epidemiol 48: , 1937–1948. |
[16] | Drinkwater E , Davies C , Spires-Jones TL ((2022) ) Potential neurobiological links between social isolation and Alzheimer’s disease risk. Eur J Neurosci 56: , 5397–5412. |
[17] | Mucke L , Masliah E , Yu GQ , Mallory M , Rockenstein EM , Tatsuno G , Hu K , Kholodenko D , Johnson-Wood K , McConlogue L ((2000) ) High-level neuronal expression of abeta 1-42 in wild-type human amyloid protein precursor transgenic mice: Synaptotoxicity without plaque formation. J Neurosci 20: , 4050–4058. |
[18] | Saito T , Matsuba Y , Mihira N , Takano J , Nilsson P , Itohara S , Iwata N , Saido TC ((2014) ) Single App knock-in mouse models of Alzheimer’s disease. Nat Neurosci 17: , 661–663. |
[19] | Hsiao K , Chapman P , Nilsen S , Eckman C , Harigaya Y , Younkin S , Yang F , Cole G ((1996) ) Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science 274: , 99–102. |
[20] | Oakley H , Cole SL , Logan S , Maus E , Shao P , Craft J , Guillozet-Bongaarts A , Ohno M , Disterhoft J , Van Eldik L , Berry R , Vassar R ((2006) ) Intraneuronal beta-amyloid aggregates,neurodegeneration, and neuron loss intransgenic mice with five familial Alzheimer’s disease mutations:Potential factors in amyloid plaque formation. J Neurosci 26: , 10129–10140. |
[21] | Jankowsky JL , Fadale DJ , Anderson J , Xu GM , Gonzales V , Jenkins NA , Copeland NG , Lee MK , Younkin LH , Wagner SL , Younkin SG , Borchelt DR ((2004) ) Mutant presenilins specifically elevate the levels of the 42residue beta-amyloid peptide in vivo: Evidence for augmentation of a42-specific gamma secretase. Hum Mol Genet 13: , 159–170. |
[22] | Cohen RM , Rezai-Zadeh K , Weitz TM , Rentsendorj A , Gate D , Spivak I , Bholat Y , Vasilevko V , Glabe CG , Breunig JJ , Rakic P , Davtyan H , Agadjanyan MG , Kepe V , Barrio JR , Bannykh S , Szekely CA , Pechnick RN , Town T ((2013) ) A transgenic alzheimer rat with plaques, tau pathology, behavioral impairment, oligomeric Aβ, and frank neuronal loss. J Neurosci 33: , 6245–6256. |
[23] | Mendez MF ((2019) ) Early-onset Alzheimer disease and its variants. Continuum (Minneap Minn) 25: , 34–51. |
[24] | Kosel F , Torres Munoz P , Yang JR , Wong AA , Franklin TB ((2019) ) Age-related changes in social behaviours in the 5xFAD mouse model of Alzheimer’s disease. Behav Brain Res 362: , 160–172. |
[25] | Cheng D , Logge W , Low JK , Garner B , Karl T ((2013) ) Novel behavioural characteristics of the APPSwe/PS1ΔE9 transgenic mouse model of Alzheimer’s disease. Behav Brain Res 245: , 120–127. |
[26] | Locci A , Orellana H , Rodriguez G , Gottliebson M , McClarty B , Dominguez S , Keszycki R , Dong H ((2021) ) Comparison of memory, affective behavior, and neuropathology in APPNLGF knock-in mice to 5xFAD and APP/PS1 mice. Behav Brain Res 404: , 113192. |
[27] | Rey CC , Robert V , Bouisset G , Loisy M , Lopez S , Cattaud V , Lejards C , Piskorowski RA , Rampon C , Chevaleyre V , Verret L ((2022) ) Altered inhibitory function in hippocampal CA2 contributes in social memory deficits in Alzheimer’s mouse model. iScience 25: , 103895. |
[28] | Chaney AM , Lopez-Picon FR , Serrière S , Wang R , Bochicchio D , Webb SD , Vandesquille M , Harte MK , Georgiadou C , Lawrence C , Busson J , Vercouillie J , Tauber C , Buron F , Routier S , Reekie T , Snellman A , Kassiou M , Rokka J , Davies KE , Rinne JO , Salih DA , Edwards FA , Orton LD , Williams SR , Chalon S , Boutin H ((2021) ) Prodromal neuroinflammatory, cholinergic and metabolite dysfunction detected by PET and MRS in the TgF344-AD transgenic rat model of AD: A collaborative multi-modal study. Theranostics 11: , 6644. |
[29] | Pietropaolo S , Delage P , Lebreton F , Crusio WE , Cho YH ((2012) ) Early development of social deficits in APP and APP-PS1 mice.. Neurobiol Aging 33: , 1002.e17–1002.e27. |
[30] | Filali M , Lalonde R , Rivest S ((2011) ) Anomalies in social behaviors and exploratory activities in an APPswe/PS1 mouse model of Alzheimer’s disease. Physiol Behav 104: , 880–885. |
[31] | Sanchez-Varo R , Sanchez-Mejias E , Fernandez-Valenzuela JJ , De Castro V , Mejias-Ortega M , Gomez-Arboledas A , Jimenez S , Sanchez-Mico MV , Trujillo-Estrada L , Moreno-Gonzalez I , Baglietto-Vargas D , Vizuete M , Davila JC , Vitorica J , Gutierrez A ((2021) ) Plaque-associated oligomeric amyloid-beta drives early synaptotoxicity in APP/PS1 mice hippocampus: Ultrastructural pathology analysis. Front Neurosci 15: , 752594. |
[32] | Richard BC , Kurdakova A , Baches S , Bayer TA , Weggen S , Wirths O ((2015) ) Gene dosage dependent aggravation of the neurological phenotype in the 5XFAD mouse model of Alzheimer’s disease. J Alzheimers Dis 45: , 1223–1236. |
[33] | Xu L , Zhou Y , Hu L , Jiang H , Dong Y , Shen H , Lou Z , Yang S , Ji Y , Ruan L , Zhang X ((2021) ) Deficits in N-methyl-D-aspartate receptorfunction and synaptic plasticity in hippocampal CA1 in APP/PS1 mousemodel of Alzheimer’s disease. Front Aging Neurosci 13: , 772980. |
[34] | Rorabaugh JM , Chalermpalanupap T , Botz-Zapp CA , Fu VM , Lembeck NA , Cohen RM , Weinshenker D ((2017) ) Chemogenetic locus coeruleus activation restores reversal learning in a rat model of Alzheimer’s disease. Brain 140: , 3023–3038. |
[35] | Minkeviciene R , Ihalainen J , Malm T , Matilainen O , Keksa-Goldsteine V , Goldsteins G , Iivonen H , Leguit N , Glennon J , Koistinaho J , Banerjee P , Tanila H ((2008) ) Age-related decrease in stimulated glutamate release and vesicular glutamate transporters in APP/PS1 transgenic and wild-type mice. J Neurochem 105: , 584–594. |
[36] | Jian C , Lu M , Zhang Z , Liu L , Li X , Huang F , Xu N , Qin L , Zhang Q , Zou D ((2017) ) miR-34a knockout attenuates cognitive deficits in APP/PS1 mice through inhibition of the amyloidogenic processing of APP. Life Sci 182: , 104–111. |
[37] | Berkowitz LE , Harvey RE , Drake E , Thompson SM , Clark BJ ((2018) ) Progressive impairment of directional and spatially precise trajectories by TgF344-Alzheimer’s disease rats in the Morris Water Task. Sci Rep 8: , 16153. |
[38] | Huang HJ , Liang KC , Ke HC , Chang YY , Hsieh-Li HM ((2011) ) Long-term social isolation exacerbates the impairment of spatial working memory in APP/PS1 transgenic mice. Brain Res 1371: , 150–160. |
[39] | Huang H , Wang L , Cao M , Marshall C , Gao J , Xiao N , Hu G , Xiao M ((2015) ) Isolation housing exacerbates Alzheimer’s disease-like pathophysiology in aged APP/PS1 mice. Int J Neuropsychopharmacol 18: , 1–10. |
[40] | Peterman JL , White JD , Calcagno A , Hagen C , Quiring M , Paulhus K , Gurney T , Eimerbrink MJ , Curtis M , Boehm GW , Chumley MJ ((2020) ) Prolonged isolation stress accelerates the onset of Alzheimer’s disease-related pathology in 5xFAD mice despite running wheels and environmental enrichment. Behav Brain Res 379: , 112366. |
[41] | He HY , Cline HT ((2019) ) What is excitation/inhibition and how is it regulated? A case of the elephant and the wisemen. J Exp Neurosci 13: , 1179069519859371. |
[42] | Zhou S , Yu Y ((2018) ) Synaptic E-I balance underlies efficient neural coding. Front Neurosci 12: , 307227. |
[43] | Bartley AF , Dobrunz LE ((2015) ) Short-term plasticity regulates the excitation/inhibition ratio and the temporal window for spike integration in CA1 pyramidal cells. Eur J Neurosci 41: , 1402–1415. |
[44] | Bhatia A , Moza S , Bhalla US ((2019) ) Precise excitation-inhibition balance controls gain and timing in the hippocampus. Elife 8: , e43415. |
[45] | Atallah BV , Scanziani M ((2009) ) Instantaneous modulation of gamma oscillation frequency by balancing excitation with inhibition. Neuron 62: , 566–577. |
[46] | Gao R , Peterson EJ , Voytek B ((2017) ) Inferring synaptic excitation/inhibition balance from field potentials. Neuroimage 158: , 70–78. |
[47] | Kann O ((2016) ) The interneuron energy hypothesis: Implications for brain disease. Neurobiol Dis 90: , 75–85. |
[48] | Amilhon B , Huh CYL , Manseau F , Ducharme G , Nichol H , Adamantidis A , Williams S ((2015) ) parvalbumin interneurons of hippocampus tune population activity at theta frequency. Neuron 86: , 1277–1289. |
[49] | Antonoudiou P , Tan YL , Kontou G , Louise Upton A , Mann EO ((2020) ) Parvalbumin and somatostatin interneurons contribute to the generation of hippocampal gamma oscillations. J Neurosci 40: , 7668–7687. |
[50] | Schlingloff D , Káli S , Freund TF , Hájos N , Gulyás AI ((2014) ) Mechanisms of sharp wave initiation and ripple generation. J Neurosci 34: , 11385. |
[51] | Ognjanovski N , Schaeffer S , Wu J , Mofakham S , Maruyama D , Zochowski M , Aton SJ ((2017) ) Parvalbumin-expressing interneurons coordinate hippocampal network dynamics required for memory consolidation. Nat Commun 8: , 15039. |
[52] | Colgin LL , Denninger T , Fyhn M , Hafting T , Bonnevie T , Jensen O , Moser M-B , Moser EI ((2009) ) Frequency of gamma oscillations routes flow of information in the hippocampus. Naturef 462: , 353–357. |
[53] | Korotkova T , Fuchs EC , Ponomarenko A , von Engelhardt J , Monyer H ((2010) ) NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron 68: , 557–569. |
[54] | Girardeau G , Benchenane K , Wiener SI , Buzsáki G , Zugaro MB ((2009) ) Selective suppression of hippocampal ripples impairs spatial memory. Nat Neurosci 12: , 1222–1223. |
[55] | Ruden JB , Dugan LL , Konradi C ((2021) ) Parvalbumin interneuron vulnerability and brain disorders. Neuropsychopharmacology 46: , 279–287. |
[56] | Calvo-Flores Guzmán B , Vinnakota C , Govindpani K , Waldvogel HJ , Faull RLM , Kwakowsky A ((2018) ) The GABAergic system as a therapeutic target for Alzheimer’s disease. J Neurochem 146: , 649–669. |
[57] | Palop JJ , Mucke L ((2016) ) Network abnormalities and interneuron dysfunction in Alzheimer disease. Nat Rev Neurosci 17: , 777–792. |
[58] | Bi D , Wen L , Wu Z , Shen Y ((2020) ) GABAergic dysfunction in excitatory and inhibitory (E/I) imbalance drives the pathogenesis of Alzheimer’s disease. Alzheimers Dement 16: , 1312–1329. |
[59] | Xu Y , Zhao M , Han Y , Zhang H ((2020) ) GABAergic inhibitory interneuron deficits in Alzheimer’s disease: Implications for treatment. Front Neurosci 14: , 660. |
[60] | Ramírez-Toranõ F , Brunã R , De Frutos-Lucas J , Rodríguez-Rojo IC , Marcos De Pedro S , Delgado-Losada ML , Gómez-Ruiz N , Barabash A , Marcos A , López Higes R , Maestú F ((2021) ) Functional connectivity hypersynchronization in relatives of Alzheimer’s disease patients: An early E/I balance dysfunction? Cereb Cortex 31: , 1201–1210. |
[61] | Hollnagel JO , Elzoheiry S , Gorgas K , Kins S , Beretta CA , Kirsch J , Kuhse J , Kann O , Kiss E ((2019) ) Early alterations in hippocampal perisomatic GABAergic synapses and network oscillations in a mouse model of Alzheimer’s disease amyloidosis. PLoS One 14: , e0209228. |
[62] | Caccavano A , Lorenzo Bozzelli P , Forcelli PA , Pak DTS , Wu JY , Conant K , Vicini S ((2020) ) Inhibitory parvalbumin basket cell activity is selectively reduced during hippocampal sharp wave ripples in a mouse model of familial Alzheimer’s disease. J Neurosci 40: , 5116–5136. |
[63] | Hijazi S , Heistek TS , Scheltens P , Neumann U , Shimshek DR , Mansvelder HD , Smit AB , van Kesteren RE ((2020) ) Early restoration of parvalbumin interneuron activity prevents memory loss and network hyperexcitability in a mouse model of Alzheimer’s disease. Mol Psychiatry 25: , 3380–3398. |
[64] | Etter G , van der Veldt S , Manseau F , Zarrinkoub I , Trillaud-Doppia E , Williams S ((2019) ) Optogenetic gamma stimulation rescues memory impairments in an Alzheimer’s disease mouse model. Nat Commun 10: , 5322. |
[65] | Verret L , Mann EO , Hang GB , Barth AMI , Cobos I , Ho K , Devidze N , Masliah E , Kreitzer AC , Mody I , Mucke L , Palop JJ ((2012) ) Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell 149: , 708–721. |
[66] | Minkeviciene R , Rheims S , Dobszay MB , Zilberter M , Hartikainen J , Fülöp L , Penke B , Zilberter Y , Harkany T , Pitkänen A , Tanila H ((2009) ) Amyloid beta-induced neuronal hyperexcitability triggers progressive epilepsy. J Neurosci 29: , 3453–3462. |
[67] | Stoiljkovic M , Kelley C , Stutz B , Horvath TL , Hajós M ((2019) ) Altered cortical and hippocampal excitability in TgF344-AD rats modeling Alzheimer’s disease pathology. Cereb Cortex 29: , 2716–2727. |
[68] | Ziyatdinova S , Gurevicius K , Kutchiashvili N , Bolkvadze T , Nissinen J , Tanila H , Pitkänen A ((2011) ) Spontaneous epileptiform discharges in a mouse model of Alzheimer’s disease are suppressed by antiepileptic drugs that block sodium channels. Epilepsy Res 94: , 75–85. |
[69] | Maestú F , de Haan W , Busche MA , DeFelipe J ((2021) ) Neuronal excitation/inhibition imbalance: Core element of a translational perspective on Alzheimer pathophysiology. Ageing Res Rev 69: , 101372. |
[70] | Targa Dias Anastacio H , Matosin N , Ooi L ((2022) ) Neuronal hyperexcitability in Alzheimer’s disease: What are the drivers behind this aberrant phenotype? Transl Psychiatry 12: , 257. |
[71] | Goutagny R , Gu N , Cavanagh C , Jackson J , Chabot JG , Quirion R , Krantic S , Williams S ((2013) ) Alterations in hippocampal network oscillations and theta-gamma coupling arise before Aβ overproduction in a mouse model of Alzheimer’s disease. Eur J Neurosci 37: , 1896–1902. |
[72] | Mondragón-Rodríguez S , Gu N , Manseau F , Williams S ((2018) ) Alzheimer’s transgenic model is characterized by very early brain network alterations and β-CTF fragment accumulation: Reversal by β-secretase inhibition. Front Cell Neurosci 12: , 121. |
[73] | Garcia-Alloza M , Robbins EM , Zhang-Nunes SX , Purcell SM , Betensky RA , Raju S , Prada C , Greenberg SM , Bacskai BJ , Frosch MP ((2006) ) Characterization of amyloid deposition in the APPswe/PS1dE9 mouse model of Alzheimer disease. Neurobiol Dis 24: , 516–524. |
[74] | Ruiter M , Herstel LJ , Wierenga CJ , Ma T ((2020) ) Reduction of dendritic inhibition in CA1 pyramidal neurons in amyloidosis models of early Alzheimer’s disease. J Alzheimers Dis 78: , 951. |
[75] | Murray AJ , Sauer JF , Riedel G , McClure C , Ansel L , Cheyne L , Bartos M , Wisden W , Wulff P ((2011) ) Parvalbumin-positive CA1 interneurons are required for spatial working but not for reference memory. Nat Neurosci 14: , 297–299. |
[76] | Hijazi S , Heistek TS , van der Loo R , Mansvelder HD , Smit AB , van Kesteren RE ((2020) ) Hyperexcitable parvalbumin interneurons render hippocampal circuitry vulnerable to amyloid beta. iScience 23: , 101271. |
[77] | Buetfering C , Allen K , Monyer H ((2014) ) Parvalbumin interneurons provide grid cell-driven recurrent inhibition in the medial entorhinal cortex. Nat Neurosci 17: , 710–718. |
[78] | Miao C , Cao Q , Moser MB , Moser EI ((2017) ) Parvalbumin and somatostatin interneurons control different space-coding networks in the medial entorhinal cortex. Cell 171: , 507-521.e17. |
[79] | Calişkan G , Müller I , Semtner M , Winkelmann A , Raza AS , Hollnagel JO , Rösler A , Heinemann U , Stork O , Meier JC ((2016) ) Identification of parvalbumin interneurons as cellular substrate of fear memory persistence. Cereb Cortex 26: , 2325–2340. |
[80] | Xia F , Richards BA , Tran MM , Josselyn SA , Takehara-Nishiuchi K , Frankland PW ((2017) ) Parvalbumin-positive interneurons mediate neocortical-hippocampal interactions that are necessary for memory consolidation. Elife 6: , e27868. |
[81] | Meira T , Leroy F , Buss EW , Oliva A , Park J , Siegelbaum SA ((2018) ) A hippocampal circuit linking dorsal CA2 to ventral CA1 critical for social memory dynamics. Nat Commun 9: , 4163. |
[82] | Okuyama T , Kitamura T , Roy DS , Itohara S , Tonegawa S ((2016) ) Ventral CA1 neurons store social memory. Science 353: , 1536–1541. |
[83] | Deng X , Gu L , Sui N , Guo J , Liang J ((2019) ) Parvalbumin interneuron in the ventral hippocampus functions as a discriminator in social memory. Proc Natl Acad Sci U S A 116: , 16583–16592. |
[84] | Domínguez S , Rey CC , Therreau L , Fanton A , Massotte D , Verret L , Piskorowski RA , Chevaleyre V ((2019) ) Maturation of PNN and ErbB4 signaling in area CA2 during adolescence underlies the emergence of PV interneuron plasticity and social memory. Cell Rep 29: , 1099–1112.e4. |
[85] | Botcher NA , Falck JE , Thomson AM , Mercer A ((2014) ) Distribution of interneurons in the CA2 region of the rat hippocampus. Front Neuroanat 8: , 104. |
[86] | Chen S , He L , Huang AJY , Boehringer R , Robert V , Wintzer ME , Polygalov D , Weitemier AZ , Tao Y , Gu M , Middleton SJ , Namiki K , Hama H , Therreau L , Chevaleyre V , Hioki H , Miyawaki A , Piskorowski RA , McHugh TJ ((2020) ) A hypothalamic novelty signal modulates hippocampal memory. Nature 586: , 270–274. |
[87] | Robert V , Therreau L , Chevaleyre V , Lepicard E , Viollet C , Cognet J , Huang AJY , Boehringer R , Polygalov D , McHugh TJ , Piskorowski RA ((2021) ) Local circuit allowing hypothalamic control of hippocampal area CA2 activity and consequences for CA1. Elife 10: , e63352. |
[88] | Piskorowski RA , Chevaleyre V ((2013) ) Delta-opioid receptors mediate unique plasticity onto parvalbumin-expressing interneurons in area CA2 of the hippocampus. J Neurosci 33: , 14567–14578. |
[89] | Loisy M , Bouisset G , Lopez S , Muller M , Spitsyn A , Duval J , Piskorowski RA , Verret L , Chevaleyre V ((2022) ) Sequential inhibitory plasticities in hippocampal area CA2 and social memory formation. Neuron 110: , 2854–2866.e4. |
[90] | Tirko NN , Eyring KW , Carcea I , Mitre M , Chao M V. , Froemke RC , Tsien RW ((2018) ) Oxytocin transforms firing mode of CA2 hippocampal neurons.. Neuron 100: , 593–608.e3. |
[91] | Raam T , McAvoy KM , Besnard A , Veenema A , Sahay A ((2017) ) Hippocampal oxytocin receptors are necessary for discrimination of social stimuli. Nat Commun 8: , 2001. |
[92] | Lin YT , Hsieh TY , Tsai TC , Chen CC , Huang CC , Hsu Sen K ((2018) ) Conditional deletion of hippocampal CA2/CA3a oxytocin receptors impairs the persistence of long-term social recognition memory in mice. J Neurosci 38: , 1218–1231. |
[93] | van den Berg M , Toen D , Verhoye M , Keliris GA ((2023) ) Alterations in theta-gamma coupling and sharp wave-ripple, signs of prodromal hippocampal network impairment in the TgF344-AD rat model. Front Aging Neurosci 15: , 1081058. |
[94] | Busche MA , Eichhoff G , Adelsberger H , Abramowski D , Wiederhold KH , Haass C , Staufenbiel M , Konnerth A , Garaschuk O ((2008) ) Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer’s disease. Science 321: , 1686–1689. |
[95] | Palop JJ , Chin J , Roberson ED , Wang J , Thwin MT , Bien-Ly N , Yoo J , Ho KO , Yu GQ , Kreitzer A , Finkbeiner S , Noebels JL , Mucke L ((2007) ) Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron 55: , 697–711. |
[96] | Amatniek JC , Hauser WA , DelCastillo-Castaneda C , Jacobs DM , Marder K , Bell K , Albert M , Brandt J , Stern Y ((2006) ) Incidence and predictors of seizures in patients with Alzheimer’s disease. Epilepsia 47: , 867–872. |
[97] | Sos KE , Mayer MI , Takács VT , Major A , Bardóczi Z , Beres BM , Szeles T , Saito T , Saido TC , Mody I , Freund TF , Nyiri G ((2020) ) Amyloid β induces interneuron-specific changes in the hippocampus of APPNL-F mice. PLoS One 15: , e0233700. |
[98] | Morrone CD , Lai AY , Bishay J , Hill ME , McLaurin JA ((2022) ) Parvalbumin neuroplasticity compensates for somatostatin impairment, maintaining cognitive function in Alzheimer’s disease. Transl Neurodegener 11: , 26. |
[99] | Mackenzie-Gray Scott CA , Pelkey KA , Caccavano AP , Abebe D , Lai M , Black KN , Brown ND , Trevelyan AJ , McBain CJ ((2022) ) Resilient hippocampal gamma rhythmogenesis and parvalbumin-expressing interneuron function before and after plaque burden in 5xFAD Alzheimer’s disease model. Front Synaptic Neurosci 14: , 857608. |
[100] | Algamal M , Russ AN , Miller MR , Hou SS , Maci M , Munting LP , Zhao Q , Gerashchenko D , Bacskai BJ , Kastanenka KV ((2022) ) Hyperactive somatostatin interneurons near amyloid plaque and cell-type-specific firing deficits in a mouse model of Alzheimer’s disease.2022.04.27. bioRxiv 489759. |
[101] | Mattson MP ((2020) ) Involvement of GABAergic interneuron dysfunction and neuronal network hyperexcitability in Alzheimer’s disease: Amelioration by metabolic switching. Int Rev Neurobiol 154: , 191–205. |
[102] | Ramos B , Baglietto-Vargas D , Rio del JC , Moreno-Gonzalez I , Santa-Maria C , Jimenez S , Caballero C , Lopez-Tellez JF , Khan ZU , Ruano D , Gutierrez A , Vitorica J ((2006) ) Early neuropathology of somatostatin/NPY GABAergic cells in the hippocampus of a PS1xAPP transgenic model of Alzheimer’s disease. Neurobiol Aging 27: , 1658–1672. |
[103] | Ali AB , Islam A , Constanti A ((2023) ) The fate of interneurons, GABAA receptor sub-types and perineuronal nets in Alzheimer’s disease. Brain Pathol 33: , e13129. |
[104] | Mahar I , Albuquerque MS , Mondragon-Rodriguez S , Cavanagh C , Davoli MA , Chabot J-G , Williams S , Mechawar N , Quirion R , Krantic S ((2017) ) Phenotypic alterations in hippocampal NPY- and PV-expressing interneurons in a presymptomatic transgenic mouse model of Alzheimer’s disease. Front Aging Neurosci 8: , 327. |
[105] | Shu S , Xu SY , Ye L , Liu Y , Cao X , Jia JQ , Bian HJ , Liu Y , Zhu XL , Xu Y ((2023) ) Prefrontal parvalbumin interneurons deficits mediate early emotional dysfunction in Alzheimer’s disease. Neuropsychopharmacology 48: , 391–401. |
[106] | Brady DR , Mufson EJ ((1997) ) Parvalbumin-immunoreactive neurons in the hippocampal formation of Alzheimer’s diseased brain. Neuroscience 80: , 1113–1125. |
[107] | Li H , Zhao J , Lai L , Xia Y , Wan C , Wei S , Liang J , Chen Y , Xu N ((2022) ) Loss of SST and PV positive interneurons in the ventral hippocampus results in anxiety-like behavior in 5xFAD mice. Neurobiol Aging 117: , 165–178. |
[108] | Giesers NK , Wirths O ((2020) ) Loss of hippocampal calretinin and parvalbumin interneurons in the 5XFAD mouse model of Alzheimer’s disease. ASN Neuro 12: , 1759091420925356. |
[109] | Zallo F , Gardenal E , Verkhratsky A , Rodríguez JJ ((2018) ) Loss of calretinin and parvalbumin positive interneurones in the hippocampal CA1 of aged Alzheimer’s disease mice. Neurosci Lett 681: , 19–25. |
[110] | Sun Y , Rimmer L , Pezze MA , Bast T , Gigg J , Harte M ((2021) ) Early-stage hippocampal hyperexcitability and impaired synaptic plasticity in the TgF344-AD rat model of Alzheimer’s disease. Alzheimers Dement 17: , e052051. |
[111] | Takahashi H , Brasnjevic I , Rutten BPF , Van Der Kolk N , Perl DP , Bouras C , Steinbusch HWM , Schmitz C , Hof PR , Dickstein DL ((2010) ) Hippocampal interneuron loss in an APP/PS1 double mutant mouse and in Alzheimer’s disease. Brain Struct Funct 214: , 145–160. |
[112] | Casas C , Sergeant N , Itier JM , Blanchard V , Wirths O , van der Kolk N , Vingtdeux V , van de Steeg E , Ret G , Canton T , Drobecq H , Clark A , Bonici B , Delacourte A , Benavides J , Schmitz C , Tremp G , Bayer TA , Benoit P , Pradier L ((2004) ) Massive CA1/2 neuronal loss with intraneuronal and N-terminal truncated Abeta42 accumulation in a novel Alzheimer transgenic model. Am J Pathol 165: , 1289–1300. |
[113] | Filice F , Vörckel KJ , Sungur AÖ , Wöhr M , Schwaller B ((2016) ) Reduction in parvalbumin expression not loss of the parvalbumin-expressing GABA interneuron subpopulation in genetic parvalbumin and shank mouse models of autism. Mol Brain 9: , 10. |
[114] | Müller M , Felmy F , Schwaller B , Schneggenburger R ((2007) ) parvalbumin is a mobile presynaptic Ca2+ buffer in the calyx of held that accelerates the decay of Ca2+ and short-term facilitation. J Neurosci 27: , 2261–2271. |
[115] | Caillard O , Moreno H , Schwaller B , Llano I , Celio MR , Marty A ((2000) ) Role of the calcium-binding protein parvalbumin in short-term synaptic plasticity. Proc Natl Acad Sci U S A 97: , 13372–13377. |
[116] | Collin T , Chat M , Lucas MG , Moreno H , Racay P , Schwaller B , Marty A , Llano I ((2005) ) Developmental changes in parvalbumin regulate presynaptic Ca2+ signaling. J Neurosci 25: , 96–107. |
[117] | Sun X , Meng X , Zhang J , Li Y , Wang L , Qin X , Sui N , Zhang Y ((2012) ) GABA attenuates amyloid toxicity by downregulating its endocytosis and improves cognitive impairment. J Alzheimers Dis 31: , 635–649. |
[118] | Martorell AJ , Paulson AL , Suk HJ , Abdurrob F , Drummond GT , Guan W , Young JZ , Kim DNW , Kritskiy O , Barker SJ , Mangena V , Prince SM , Brown EN , Chung K , Boyden ES , Singer AC , Tsai LH ((2019) ) Multi-sensory gamma stimulation ameliorates Alzheimer’s-associated pathology and improves cognition. Cell 177: , 256–271.e22. |
[119] | Iaccarino HF , Singer AC , Martorell AJ , Rudenko A , Gao F , Gillingham TZ , Mathys H , Seo J , Kritskiy O , Abdurrob F , Adaikkan C , Canter RG , Rueda R , Brown EN , Boyden ES , Tsai LH ((2016) ) Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature 540: , 230–235. |
[120] | Ebert A , Brüne M ((2018) ) Oxytocin and social cognition. Curr Top Behav Neurosci 35: , 375–388. |
[121] | Jones C , Barrera I , Brothers S , Ring R , Wahlestedt C ((2017) ) Oxytocin and social functioning. Dialogues Clin Neurosci 19: , 193–201. |
[122] | Bielsky IF , Young LJ ((2004) ) Oxytocin, vasopressin, and social recognition in mammals. Peptides 25: , 1565–1574. |
[123] | Yoshida M , Takayanagi Y , Inoue K , Kimura T , Young LJ , Onaka T , Nishimori K ((2009) ) Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. J Neurosci 29: , 2259–2271. |
[124] | Hitti FL , Siegelbaum SA ((2014) ) The hippocampal CA2 region is essential for social memory. Nature 508: , 88–92. |
[125] | Lin YT , Hsu K Sen ((2018) ) Oxytocin receptor signaling in the hippocampus: Role in regulating neuronal excitability, network oscillatory activity, synaptic plasticity and social memory. Prog Neurobiol 171: , 1–14. |
[126] | Knobloch HS , Grinevich V ((2014) ) Evolution of oxytocin pathways in the brain of vertebrates. Front Behav Neurosci 8: , 31. |
[127] | Bakos J , Srancikova A , Havranek T , Bacova Z ((2018) ) Molecular mechanisms of oxytocin signaling at the synaptic connection. Neural Plast 2018: , 4864107. |
[128] | Jurek B , Neumann ID ((2018) ) The oxytocin receptor: From intracellular signaling to behavior. Physiol Rev 98: , 1805–1908. |
[129] | Lin YT , Huang CC , Hsu Sen K ((2012) ) Oxytocin promotes long-term potentiation by enhancing epidermal growth factor receptor-mediated local translation of protein kinase Mζ. J Neurosci 32: , 15476–15488. |
[130] | Serrano P , Friedman EL , Kenney J , Taubenfeld SM , Zimmerman JM , Hanna J , Alberini C , Kelley AE , Maren S , Rudy JW , Yin JCP , Sacktor TC , Fenton AA ((2008) ) PKMzeta maintains spatial, instrumental, and classically conditioned long-term memories. PLoS Biol 6: , 2698–2706. |
[131] | Tsai TC , Fang YS , Hung YC , Hung LC , Hsu Sen K ((2022) ) A dorsal CA2 to ventral CA1 circuit contributes to oxytocinergic modulation of long-term social recognition memory. J Biomed Sci 29: , 50. |
[132] | Lopatina OL , Komleva YK , Gorina YV , Olovyannikova RY , Trufanova LV , Hashimoto T , Takahashi T , Kikuchi M , Minabe Y , Higashida H , Salmina AB ((2018) ) Oxytocin and excitation/inhibition balance in social recognition. Neuropeptides 72: , 1–11. |
[133] | Zaninetti M , Raggenbass M ((2000) ) Oxytocin receptor agonists enhance inhibitory synaptic transmission in the rat hippocampus by activating interneurons in stratum pyramidale. Eur J Neurosci 12: , 3975–3984. |
[134] | Owen SF , Tuncdemir SN , Bader PL , Tirko NN , Fishell G , Tsien RW ((2013) ) Oxytocin enhances hippocampal spike transmission by modulating fast-spiking interneurons. Nature 500: , 458–462. |
[135] | Mühlethaler M , Charpak S , Dreifuss JJ ((1984) ) Contrasting effects of neurohypophysial peptides on pyramidal and non-pyramidal neurones in the rat hippocampus. Brain Res 308: , 97–107. |
[136] | Cochran DM , Fallon D , Hill M , Frazier JA ((2013) ) The role of oxytocin in psychiatric disorders: A review of biological and therapeutic research findings. Harv Rev Psychiatry 21: , 219–247. |
[137] | Jacobson JD , Ellerbeck KA , Kelly KA , Fleming KK , Jamison TR , Coffey CW , Smith CM , Reese RM , Sands SA ((2014) ) Evidencefor alterations in stimulatory G proteins andoxytocin levels in children with autism. Psychoneuroendocrinology 40: , 159–169. |
[138] | Johnson EG , Kuiper W , Ahmed RM , Halliday GM , Burrell JR , Hodges JR , Guastella AJ , Piguet O , Kumfor F ((2022) ) Plasma oxytocin is not associated with social cognition or behavior in frontotemporal dementia and Alzheimer’s disease syndromes. Dement Geriatr Cogn Disord 51: , 241–248. |
[139] | Wierda M , Goudsmit E , Van Der Woude PF , Purba JS , Hofman MA , Bogte H , Swaab DF ((1991) ) Oxytocin cell number in the human paraventricular nucleus remains constant with aging and in Alzheimer’s disease. Neurobiol Aging 12: , 511–516. |
[140] | Popik P , Vetulani J , van Ree JM ((1992) ) Low doses of oxytocin facilitate social recognition in rats. Psychopharmacology (Berl) 106: , 71–74. |
[141] | Ferguson JN , Aldag JM , Insel TR , Young LJ ((2001) ) Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci 21: , 8278–8285. |
[142] | Neumann ID , Maloumby R , Beiderbeck DI , Lukas M , Landgraf R ((2013) ) Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology 38: , 1985–1993. |
[143] | Takahashi J , Yamada D , Ueta Y , Iwai T , Koga E , Tanabe M , Oka JI , Saitoh A ((2020) ) Oxytocin reverses Aβ-induced impairment of hippocampal synaptic plasticity in mice. Biochem Biophys Res Commun 528: , 174–178. |
[144] | Takahashi J , Ueta Y , Yamada D , Sasaki-Hamada S , Iwai T , Akita T , Yamashita C , Saitoh A , Oka JI ((2022) ) Intracerebroventricular administration of oxytocin and intranasaladministration of the oxytocin derivative improve β-amyloidpeptide (25-35)-induced memory impairment in mice. Neuropsychopharmacol Rep 42: , 492–501. |
[145] | El-Ganainy SO , Soliman OA , Ghazy AA , Allam M , Elbahnasi AI , Mansour AM , Gowayed MA ((2022) ) Intnasaral oxytocin attenuates cognitive impairment, β-amyloid burden and tau deposition in female rats with Alzheimer’s disease: Interplay of ERK1/2/GSK3β/caspase-3. Neurochem Res 47: , 2345–2356. |
[146] | Selles MC , Fortuna JTS , Faria de YPR , Longo BM , Froemke RC , Chao MV , Ferreira ST (2022) Oxytocin attenuates microglial activation and restores social and non-social memory in the APP/PS1 mouse model of Alzheimer’s disease. bioRxiv 2022.05.07.491031. |
[147] | Lin YT , Chen CC , Huang CC , Nishimori K , Hsu Sen K ((2017) ) Oxytocin stimulates hippocampal neurogenesis via oxytocin receptor expressed in CA3 pyramidal neurons. Nat Commun 8: , 537. |
[148] | Yao Y , Kelly MT , Sajikumar S , Serrano P , Tian D , Bergold PJ , Frey JU , Sacktor TC ((2008) ) PKMζ maintains late long-term potentiation by N-ethylmaleimide-sensitive factor/GluR2-dependent trafficking of postsynaptic AMPA receptors. J Neurosci 28: , 7820–7827. |
[149] | Raggenbass M , Tribollet E , Dubois-Dauphin M , Dreifuss JJ ((1989) ) Correlation between oxytocin neuronal sensitivity and oxytocin receptor binding: An electrophysiological and autoradiographical study comparing rat and guinea pig hippocampus. Proc Natl Acad Sci U S A 86: , 750–754. |



