Posttraumatic Stress and Traumatic Brain Injury: Cognition, Behavior, and Neuroimaging Markers in Vietnam Veterans
Abstract
Background:
Posttraumatic stress disorder (PTSD) and traumatic brain injury (TBI) are common in Veterans and linked to behavioral disturbances, increased risk of cognitive decline, and Alzheimer’s disease.
Objective:
We studied the synergistic effects of PTSD and TBI on behavioral, cognitive, and neuroimaging measures in Vietnam war Veterans.
Methods:
Data were acquired at baseline and after about one-year from male Veterans categorized into: PTSD, TBI, PTSD+TBI, and Veteran controls without PTSD or TBI. We applied manual tractography to examine white matter microstructure of three fiber tracts: uncinate fasciculus (N = 91), cingulum (N = 87), and inferior longitudinal fasciculus (N = 95). ANCOVAs were used to compare Veterans’ baseline behavioral and cognitive functioning (N = 285), white matter microstructure, amyloid-β (N = 230), and tau PET (N = 120). Additional ANCOVAs examined scores’ differences from baseline to follow-up.
Results:
Veterans with PTSD and PTSD+TBI, but not Veterans with TBI only, exhibited poorer behavioral and cognitive functioning at baseline than controls. The groups did not differ in baseline white matter, amyloid-β, or tau, nor in behavioral and cognitive functioning, and tau accumulation change. Progression of white matter abnormalities of the uncinate fasciculus in Veterans with PTSD compared to controls was observed; analyses in TBI and PTSD+TBI were not run due to insufficient sample size.
Conclusions:
PTSD and PTSD+TBI negatively affect behavioral and cognitive functioning, while TBI does not contribute independently. Whether progressive decline in uncinate fasciculus microstructure in Veterans with PTSD might account for cognitive decline should be further studied. Findings did not support an association between PTSD, TBI, and Alzheimer’s disease pathology based on amyloid and tau PET.
INTRODUCTION
Posttraumatic stress disorder (PTSD) and traumatic brain injury (TBI) are highly prevalent among military Veterans [1, 2] and are associated with short- and long-term behavioral disturbances and cognitive decline [3–5]. Interestingly, many long-term symptoms related to PTSD and TBI can also be observed in individuals with Alzheimer’s disease (AD). Overlapping symptoms include cognitive decline in attention, memory, and language performance [6, 7], neuropsychiatric symptoms such as depression, apathy, or agitation [8, 9], and impairment in activities of daily living [6].
Researchers have speculated that common underlying neuropathologies can explain the symptomatic overlap between PTSD, TBI, and AD [10–14]. Specifically, the accumulation of amyloid-β (Aβ) plaques and tau neurofibrillary tangles have been suggested as two of the mechanisms underlying PTSD, TBI, and AD [15, 16]. Neuritic Aβ plaques and tau deposits are indeed hallmarks of AD pathology [17, 18]. Animal research showed that psychological and physiological stress leads to Aβ genesis, proposing a potential link between Aβ plaques, PTSD, and TBI [19–22]. PTSD studies in humans, however, are limited and do not support the idea of an accumulation of Aβ plaques or tau in PTSD [23–26]. In vivo studies in TBI presented mixed results, with some amyloid and tau positron emission tomography (amyloid-PET and tau-PET) studies reporting increased Aβ and tau after TBI [27, 28]. Nevertheless, others did not find Aβ plaques and tau accumulation in individuals who experienced a TBI, implying that the effect might depend on individual risk factors such as genetic or medical conditions and the type of exposure and timing of TBI [25, 29]. A recent study also found no association between remote mild TBI and cortical Aβ burden [30].
Recent studies also suggested that common white matter pathologies might underly AD, PTSD, and TBI. Diffusion magnetic resonance imaging (dMRI) studies showed that white matter microstructural abnormalities are present in early stages of AD, therefore models using dMRI may provide biomarkers of white matter neurodegeneration in the earliest stages of AD progression [31–33]. Notably, the same fiber tracts related to AD development are also impaired in individuals with PTSD or TBI [34–43]. While findings are somehow inconsistent [44, 45], the uncinate fasciculus (UF), the cingulum (CI), and the inferior longitudinal fasciculus (ILF) have all been associated with cognitive decline and aging in PTSD, TBI, and AD [34–36, 38, 40, 41, 46–49]. Additionally, the UF and CI were found to be the most important tracts in distinguishing groups with PTSD and trauma-exposed controls [50]. The UF connects the anterior temporal lobe with the lateral orbitofrontal cortex and is involved in emotion and memory regulation [51, 52]. The CI stretches from the orbital frontal cortex, along the corpus callosum, to the temporal lobe and pole and is involved in executive control, episodic memory, and emotion [53]. The ILF connects the occipital and temporal lobes and is critical for visual memory, perception, reading, and language functions [51].
While PTSD, TBI, and AD share some neuropathological and clinical features, it remains elusive whether PTSD [13] and TBI [25, 29, 54] predispose to AD development. A recent meta-analysis did not find a relationship between PTSD diagnosis and subsequent dementia development [55]. Similarly, prospective studies failed to detect associations between TBI and autopsy-confirmed AD [29, 54]. On the other hand, there is evidence showing that Veterans who experience psychological and physical trauma are at increased risk of developing AD [56, 57]. These findings demonstrate that we are far from understanding the link between PTSD, TBI, and vulnerability for AD, and further research is needed considering the increasing number of aging individuals in general and aging Veterans in particular.
The Department of Defense (DOD) funded a project involving Vietnam war Veterans as part of the Alzheimer’s Disease Neuroimaging Initiative (ADNI) to meet this critical need (http://adni.loni.usc.edu). AD markers were measured in Veterans decades after trauma exposure twice: at baseline and after a one-year follow-up period [58]. A previous cross-sectional ADNI-DOD study revealed lower cognitive functioning in Veterans with PTSD, TBI, and PTSD+TBI compared to Veteran controls; however, impairments in cognitive functioning were not related to Aβ deposition [25]. In that study, when using a 1.11 cut-off for amyloid positivity, the PTSD group had lower odds of amyloid positivity compared to controls, while the TBI group did not [25]. Another recent study from the ADNI-DOD group found no evidence of increased Aβ, tau, or neurodegeneration biomarkers in either TBI or PTSD [59]. Nevertheless, other ADNI-DOD studies reported a negative effect of PTSD and TBI on white matter microstructure throughout the brain and tau pathologies [60, 61].
The present study builds on previous work [25, 59] to achieve two primary goals. First, we compare baseline measures of behavioral functioning, cognition, and neuroimaging markers that have previously been related to AD between Veterans with PTSD, TBI, PTSD+TBI, and Veteran controls. Next, we examine longitudinal changes in measurements over the one-year follow-up period to test for signs of progressive aging in Veterans with PTSD, TBI, PTSD+TBI, and Veteran controls. The current study extends previous ADNI-DOD studies by analyzing dMRI data using a methodology which allows a more sensitive reconstruction of white matter tracts as well as reporting on different neurobehavioral measures.
METHODS
Study design and the ADNI
The present study used cross-sectional data and for a subset longitudinal data obtained from a multicenter trial of the ADNI database, launched by the DOD in 2012 to study dementia risk factors in Veterans. Data were downloaded in April 2020. All data available in this study have been previously collected by the ADNI-DOD group. Our contribution has been in analyzing the diffusion-weighted images using a new methodology as described in the Diffusion MRI Image Processing section.
Vietnam War Veterans of age 60–80 years old were identified and contacted through Veterans Affairs records. They underwent an initial telephone screening, if eligible a clinical interview, and if still eligible they underwent baseline and follow-up visits at one of the DOD clinic sites. Study procedures, including the recruitment process, have already been published elsewhere (ADNI-DOD Procedures Manual, http://www.adni-info.org). Assessments for cognitive, behavioral, dMRI, and amyloid- and tau-PET data have been conducted at baseline and for cognitive, behavioral, dMRI, and tau-PET after a one-year follow-up. The follow-up period varied among individuals and type of assessment. To reduce this variability we removed extreme cases (corresponding to the 3rd quartile + 3* interquartile range and 1st quartile – 3* interquartile range), resulting in the inclusion of participants with a time interval between 7.5 months and 1.6 years. The distribution of time intervals for each measure is reported in the Results section.
Participants
Three hundred and fifteen male Vietnam war Veterans between 60–80 years of age were included in the ADNI-DOD study. According to the ADNI-DOD protocol, participants were excluded if they reported a history of psychosis, bipolar disorder, a history of alcohol or substance abuse within the past five years (Diagnostic and Statistical Manual, Version IV, Text Revision, DSM IV-TR criteria); neurological disorders in the past five years and unstable (<four months) somatic conditions (e.g., cardiovascular diseases, hepatic, renal, pulmonary, metabolic diseases, or cancer). Moreover, participants were excluded when contraindications for PET (i.e., history of severe drug allergy or hypersensitivity) or MRI (i.e., aneurysm clips, metal implants, claustrophobia) applied.
Group-specific inclusion and exclusion criteria are described below:
1) PTSD participants had to meet the criteria for current or lifetime PTSD according to the Structured Clinical Interview 1 (SCID 1) of the DSM IV-TR, a minimum current Clinician Administered PTSD Scale (CAPS) score of 50, and symptoms related to the Vietnam war trauma, which were identified and verified via medical records and telephone assessments. Participants of this group were excluded if they had a documented history of TBI defined as in group 2.
2) TBI participants were required to have a documented history of non-penetrating TBI acquired during the military period in Vietnam. TBI history was identified from the DOD or Veterans Affairs records. Considering that the Glasgow Coma Scale was not available during the Vietnam war, the study coordinators used an operational definition of TBI based on diagnostic codes potentially related to TBI and available in medical records of the time, such as brain hemorrhage and traumatic brain disease (the specific TBI-related diagnostic codes can be found in Supplementary Table 1 of Weiner et al. (2023) [59]). Their operational definition also entailed non-penetrating head injury with amnesia, and/or loss of consciousness for 5 min, and/or alteration of mental state for > 24 h (also see ADNI-DOD protocol [62]). Participants of this group were excluded if they met PTSD diagnostic criteria (current or lifetime) defined as for group 1.
3) Participants of the PTSD+TBI group had to have both PTSD (diagnosed with SCID or CAPS) and TBI (identified from DOD or Veterans Affairs records) following the criteria for PTSD and TBI described in groups 1 and 2.
4) Veteran control participants were required to have neither a history of PTSD nor of TBI.
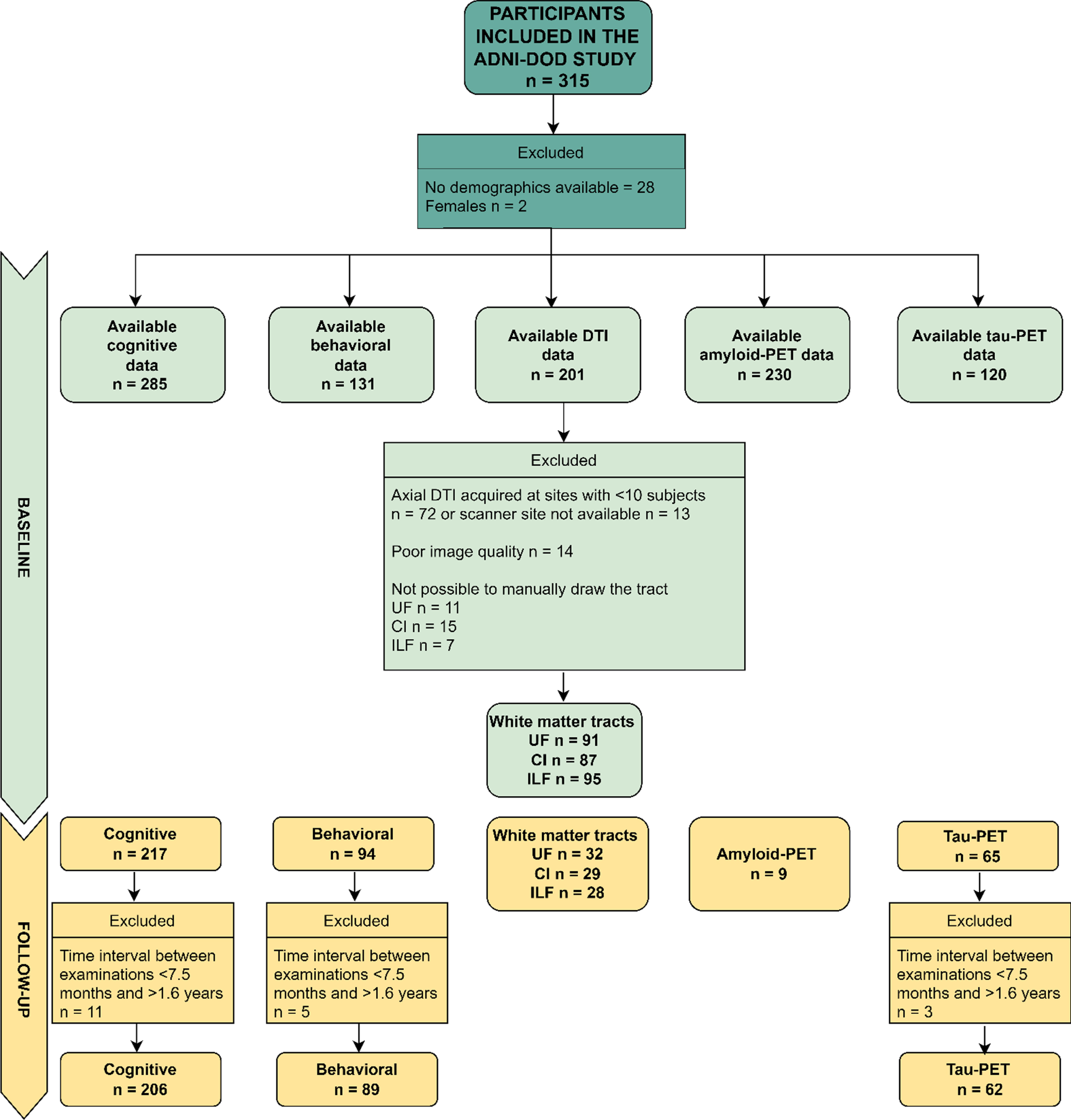
We excluded females (only 2 participants) and everyone with no demographic data for the present study. In addition, dMRI data were excluded if acquired at sites where less than ten participants had been scanned, leading to a smaller sample than implied in the recruitment protocol. This approach allowed us to more robustly correct for site differences in the statistical analyses, since the usage of different scanners and protocols has been previously shown to introduce significant variability in results [63, 64]. Considering that the sample varies for each analysis, we refer the reader to Fig. 1 which shows the available data utilized in each analysis run in the present study. Participants were classified by the ADNI-DOD team (file “VAELG.csv”) into four groups: 1) Veterans with PTSD, 2) Veterans with TBI, 3) Veterans with PTSD+TBI, and 4) Veterans without a history of TBI or PTSD (Veteran controls).
Demographics and clinical measures
Sample characteristics, including age, education, lifetime CAPS score, genotype, ethnicity, race, primary language, current work status, handedness, marital status, and psychiatric medication use, were assessed.
Cognitive and behavioral assessment
Trained psychometricians administered cognitive tests and behavioral assessment tools following standardized procedures. Cognitive tests assessed the domains of confrontation naming (Boston Naming Test, BNT [65]), executive functioning (Trails Making Test B-A, TMT B-A [66]), and episodic memory (30-minutes delay Auditory Verbal Learning Test, AVDEL30 [67]). The behavioral assessment consisted of assessing neuropsychiatric disturbances (delusions, hallucinations, agitation, dysphoria, anxiety, apathy, irritability, euphoria, disinhibition, aberrant motor behavior, night-time behavior disturbances, appetite and eating abnormalities) measured using the Neuropsychiatric Inventory (NPI) total score (calculated adding up all domain scores which were obtained multiplying frequency by severity) [68] and functioning in activities of daily living evaluated with the Functional Assessment Questionnaire (FAQ) [69]. Tests’ raw scores were used. Higher scores on the BNT and AVDEL30 and lower scores on the TMT B-A indicate better cognitive performance. Higher scores on the NPI and FAQ indicate worse behavioral functioning.
PET and MRI neuroimaging
PET acquisition
Amyloid-PET images were acquired using the [18F]-AV45 radiotracer (florbetapir) and tau-PET using [18F]-AV1451 (flortaucipir). Injection (dosage of 370 MBq, 10 mCi±10% bolus) was followed by a resting-uptake phase of 50 min for [18F]-AV45 and 75 min for [18F]-AV1451. Participants then entered the tomograph, and four emission frames of 5 min each were acquired for amyloid-PET. For tau-PET, six frames of 5-min duration were performed. Amyloid-PET images were 3D reconstructed using Iterative (fully 3D Iteration; four iterations; 20 subsets) with a grid of 128×128, FOV: 256×256 mm, slice thickness: 3.27 mm [70]. This study used the amyloid and tau PET data previously analyzed by the Jagust laboratory at the University of California Berkeley. For amyloid-PET we used the file “UCBERKELEYAV45_20190808” available on the ADNI database. For all statistical analyses the following areas were selected to measure brain Aβ: frontal (weighted florbetapir mean in frontal regions, “FRONTAL_SUVR”), cingulate (weighted florbetapir mean in anterior/posterior cingulate regions, “CINGULATE_SUVR”), parietal (Weighted florbetapir mean in lateral parietal regions, “PARIETAL_SUVR”), temporal (Weighted florbetapir mean in lateral temporal regions, “TEMPORAL_SUVR”), and composite florbetapir cortical standardized uptake value ratio, SUVR (“COMPOSITE_SUVR, Summary florbetapir cortical SUVR. Weighted florbetapir mean in frontal, cingulate, parietal, and temporal regions, defined by Freesurfer”). For tau-PET, we used a summary measure of the temporal region from the file “UCBERKELEYAV1451_MRIFREE_20210506” (temporal flortaucipir standardized uptake value ratio, “METATEMPORAL_SUVR”, weighted AV1451 mean of temporal summary region, with regions defined by neuromorphometrics and listed elsewhere [71]). The PET data available on the ADNI database is already intensity normalized; although Jagust’s laboratory recommends to normalize again the SUVRs with a reference region, as explained in details elsewhere [73]. In Supplementary Table 3, we provide results also for composite Aβ and tau SUVR relative to a reference region.
Fig.1
Flow Diagram of Available Data Used in the Current Study (Cognitive and Behavioral Data, Axial DTI, Amyloid-PET, Tau-PET at Baseline and Follow-up). Axial DTI, Axial Diffusion Tensor Imaging (as named in the ADNI data repository); CI, cingulum ILF, inferior longitudinal fasciculus; UF, uncinate fasciculus; PET, positron emission tomography. The limited sample of Amyloid-PET data at follow-up is due to budget restrictions that did not allow the ADNI-DOD team to repeat the florbetapir examinations as reported in their protocol (https://adni.loni.usc.edu/wp-content/uploads/2013/09/DOD-ADNI-IRB-Approved-Final-protocol-08072012.pdf, page 39).
MRI acquisition
MRI data were obtained using 3 Tesla scanners with standardized procedures across different ADNI sites (N = 24, this number corresponds to the institutions where the MRI acquisition took place and not to the clinical ADNI site). The current study used axial diffusion tensor imaging (“Axial DTI”) scans. Data was obtained using axial spin-echo sequence (TR/TE=9050 ms/minimum, matrix = 256×256×59, resolution = 1.37×1.37×2.7 mm35, and b-value=5×b0 + 41 directions with b = 1000 s/mm2). Further information on MRI acquisition is available in the DOD ADNI procedures manual (Version 3.0 Augustus 28, 2017) [72].
Diffusion MRI image processing
Pre-processing
Pre-processing steps were performed using an in-house pre-processing pipeline [73], correcting for head motion, eddy current distortion, and generating brain masks. Image quality was visually checked for artifacts using 3D Slicer [74], leading to the exclusion of fourteen participants (five controls, three PTSD+TBI, three TBI, and three PTSD). Brain masks were visually inspected and manually corrected where necessary using 3D Slicer.
Whole brain tractography
We reconstructed fiber pathways utilizing whole-brain unscented Kalman Filter (UKF) tractography [75]. This method reconstructs multiple fiber orientations within a voxel and is robust to noise [76, 77]. Moreover, UKF tractography can support multiple dMRI models, including single and multi-tensor models, free water (FW) imaging, or neurite orientation dispersion and density imaging (NODDI). For this study, we used a 2-tensor plus free water model (2 tensor + free water model, 1 seed per voxel, minimum seed fractional anisotropy (FA)=0.18, terminating FA = 0.15, terminating mean signal = 0.10), which has been shown to provide accurate fiber tracking [77, 78]. This approach was chosen since UKF tractography has been shown to be one of the most accurate tractography techniques especially compared to single-tensor methods [77, 79, 80]. In short, the orientation of a local fiber is traced using the estimation at the previous position to direct the estimation at the current position. Kalman filter provides a recursive estimation which greatly improves the accuracy of fiber orientations [74].
Fiber bundle extraction
The UF, CI, and ILF of both hemispheres were selected as tracts of interest. A description of the three tracts and their function is reported in Table 1. The tracts were manually extracted following procedures outlined elsewhere [81, 82]. Briefly, individual fibers from full-brain tractograms were identified as part of a tract of interest if they traverse manually defined inclusionary regions but do not traverse exclusionary regions. The rationale for choosing manual tracts of interest estimation was that automated tract identification is often anatomically inaccurate in populations with significant damage to connections [83]. While manual approaches can be impractical with large cohorts and rely on human expertise, manual fiber bundle identification results in low values of true negatives [82] and has commonly been used with patient populations [84–86].
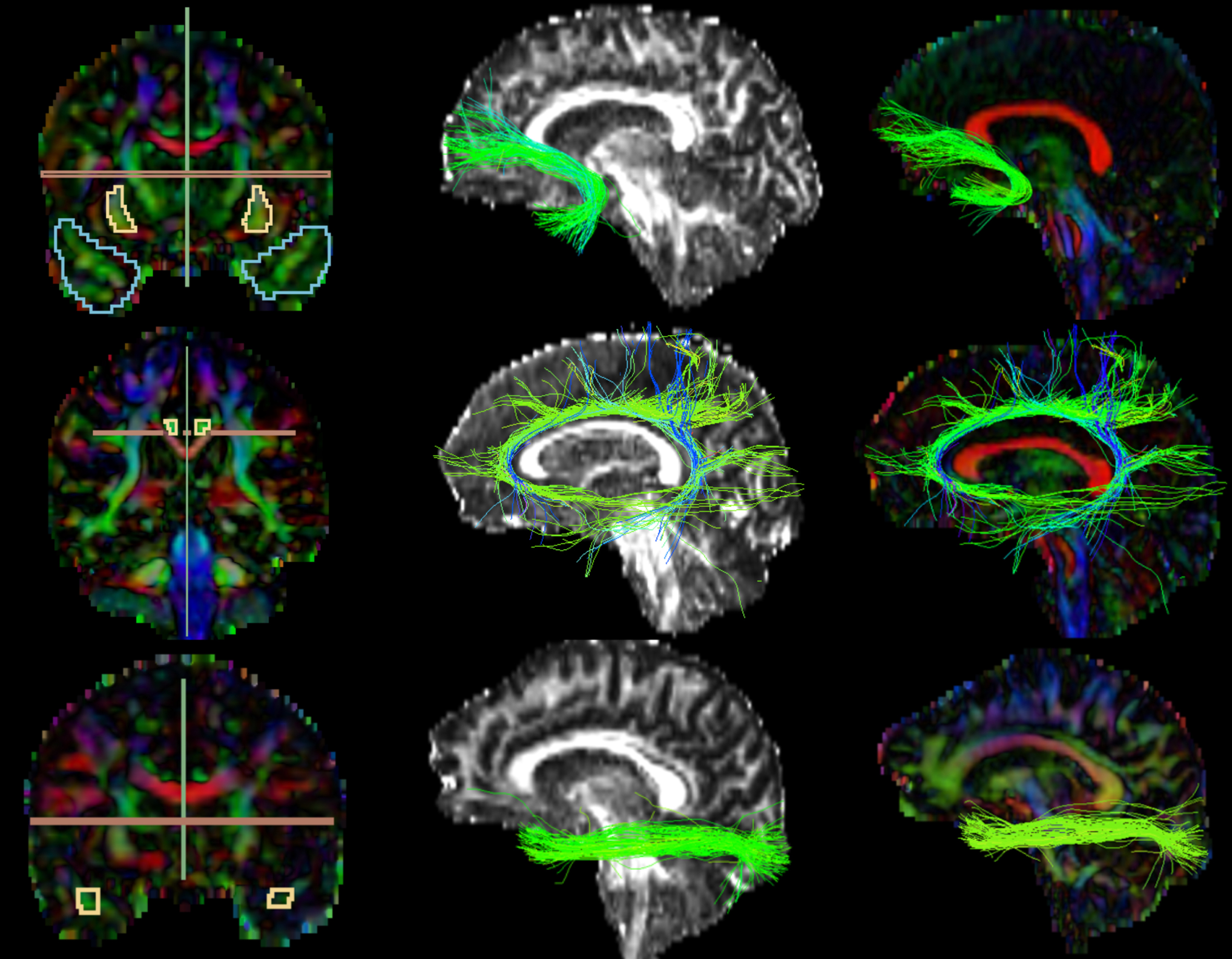
FA and color-by-orientation maps were calculated from the pre-processed dMRI data and used for the manual delineation of inclusionary and exclusionary areas according to Catani & de Schotten’s (2008) rules [81]. Specifically, for the UF, two inclusion areas (external capsule and temporal areas) and three exclusion areas (in the mid-sagittal, mid-coronal, and mid-axial slice) were drawn (Fig. 2, first row). For the CI, one inclusion region of interest (ROI) and two exclusion areas (in the axial and sagittal slice) (Fig. 2, second row). For the ILF, two inclusion ROIs (occipital and temporal areas) and two exclusion areas (in the mid-sagittal and axial slice) were drawn (Fig. 2, third row). Following the manual drawings, fiber bundles were extracted using the “Tractography ROI Selection” function in 3D Slicer with the whole-brain tractography as input and the manual drawings as label map. To establish the reliability of the procedure, three raters independently performed manual drawing delineation on four study cases.
dMRI measure extraction
The UKF tractography algorithm estimates parameters describing the 2 tensor+free water model for each point along each fiber tract. FW-corrected FA is a more accurate measure compared to traditional FA, especially when modeling tissue structure in aging populations [87], where atrophy and thus partial volume effects are more prevalent [78, 88–90].
We extracted the mean FA values of the primary tensor of each fiber bundle using the “Tractography Measurements” module of 3D Slicer and averaged left and right hemisphere measures. FA is the most widely used diffusion measure and is considered sensitive to microstructural changes, such as myelinization and axonal density.
Table 1
Extracted Tracts and Relative Function
| Tract | Location and function |
| Uncinate Fasciculus | The UF is a ventral associative bundle that connects the anterior temporal lobe with the lateral orbitofrontal cortex [146, 147]. It is considered (i) part of the limbic system [146], (ii) involved in emotion and memory [51], and (iii) object and face naming [52]. |
| Cingulate | The CI is a medial associative bundle that runs from the orbital frontal cortex, along the corpus callosum, to the temporal lobe and pole [146]. It is part of the limbic system and involved in executive control, episodic memory, emotion, and pain [53]. |
| Inferior Longitudinal Fasciculus | The ILF is a longitudinal bundle connecting the occipital and temporal lobes [146]. It is involved in face recognition [148], visual perception [149], reading [150], visual memory [151], and other language functions [51]. |
Fig. 2
Extracted Tracts. The first images on the left column partially represent the manual drawings of the inclusion and exclusion areas; 1st row) Uncinate Fasciculus: blue (temporal area) and yellow (external capsule) inclusion, green and red exclusion; 2nd row) Cingulate: yellow inclusion, red and green exclusion; 3rd row) Inferior Longitudinal Fasciculus: yellow inclusion (temporal area; occipital not shown in the picture), green and red exclusion. The images on the second and third columns represent the extracted tracts shown over the FA and color-by-orientation maps, respectively.
Fiber bundle extraction reliability
We measured the reliability of the manual tracts of interest definition procedure by having the primary rater and two additional raters perform tract of interest tracing in four study cases. Inter-rater reliability for the three tracts was calculated using a two-ways interclass correlation (ICC) with absolute agreement. The guidelines by Koo & Li (2016) were used to define the agreement, with ICC < 0.50 meaning poor agreement, 0.50–0.75 moderate, 0.75–0.90 good, >0.90 excellent. Inter-rater reliability resulted to be good or excellent for FA (UF: ICC = 0.80, CI: ICC = 0.82, ILF: ICC = 0.95).
Statistical analysis
Statistical analyses were performed using the Statistical Package for Social Sciences (SPSS) Version 27 [91]. Means, standard deviations, and one-way analyses of variance (ANOVA) were computed for continuous variables; percentages and chi-square tests for categorical variables.
Group comparisons: cognitive, behavioral, and neuroimaging measures
To test for group differences (PTSD versus TBI versus PTSD+TBI versus Veteran controls) in cognitive functioning (BNT, TMT B-A, AVDEL30) and neuroimaging measures (white matter: UF, CI, and ILF FA; amyloid and tau PET: composite florbetapir cortical SUVR and flortaucipir temporal SUVR) twelve analyses of covariance (ANCOVAs) were conducted. For differences in behavioral functioning (NPI, FAQ), two Kruskall-Wallis tests were conducted to account for the skewness of the data (skewness was respectively 3.20 and 2.11); for all other analyses skewness lied between –2 and+2 [92] Lower scores on the BNT and AVDEL30, higher scores on the TMT B-A, NPI, and FAQ, decreased white matter FA, and higher composite florbetapir cortical SUVR and flortaucipir temporal SUVR were expected in Veterans with PTSD and/or TBI groups compared to the Veteran controls.
Group comparisons: cognitive, behavioral, and neuroimaging differences (follow-up -bBaseline)
To test for group comparisons in score differences between baseline and follow-up in cognitive functioning (BNT, TMT B-A, AVDEL30), behavioral functioning (NPI, FAQ), and UF, CI, and ILF white matter FA, eight ANCOVA models were implemented. White matter measures were compared only between Veterans with PTSD and Veteran controls due to the small sample size in the TBI and PTSD+TBI groups. Moreover, we did not compare composite florbetapir cortical SUVR difference scores between groups since follow-up measures were only available for nine participants due to budget restrictions as noted in Fig. 1. Scores’ raw differences were calculated (follow-up – baseline); scatterplots displaying the association between score differences and time interval are reported in Supplementary Figures 2 and 3. A negative score difference was expected in Veterans with PTSD and/or TBI for all dependent variables, except for a positive difference in TMT B-A, NPI, FAQ, and flortaucipir temporal SUVR, as compared to Veteran controls.
An overview of the statistical approach can be found in Supplementary Figure 1 and Supplementary Table 1. Note that analyses may have different sample sizes due to missing data. All ANCOVA models were corrected for age, education, and scanner site. Groups did not differ in other demographics (ethnicity, racial category, APOE ɛ4+) accordingly, these variables were not included as covariates. All models were corrected for multiple comparisons, setting a false discovery rate at 5% using the Benjamini-Hochberg method and considering a corrected p < 0.05 as significant.
RESULTS
Demographic and sample characteristics
A summary of the sample characteristics is displayed in Table 2. The one-way ANOVAs revealed statistically significant between-group differences in age (Veteran controls significantly older than PTSD), education (PTSD significantly lower education compared to Veterans with TBI and controls, and Veteran controls significantly higher than Veterans with PTSD+TBI), and CAPS score (PTSD having the highest score). Chi-square tests showed the lowest use of psychiatric medications among Veteran controls and the highest among Veterans with PTSD.
Of the sample with complete cognitive data at baseline, 236 participants were cognitively normal, 49 had a diagnosis of MCI (15 PTSD, 9 TBI, 23 PTSD+TBI, and 2 controls), and none had dementia. At month twelve, 178 were cognitively normal and 37 had MCI (11 PTSD, 9 TBI, 16 PTSD+TBI, and 1 control). Of the sample with available DTI data (considering the ILF sample), 90 were cognitively normal, 5 had a diagnosis of MCI (2 PTSD, 3 TBI, 1 PTSD+TBI, 0 controls), and none had dementia (this data was retrieved from the “DXSUM” spreadsheet).
Group differences in cognitive and behavioral functioning
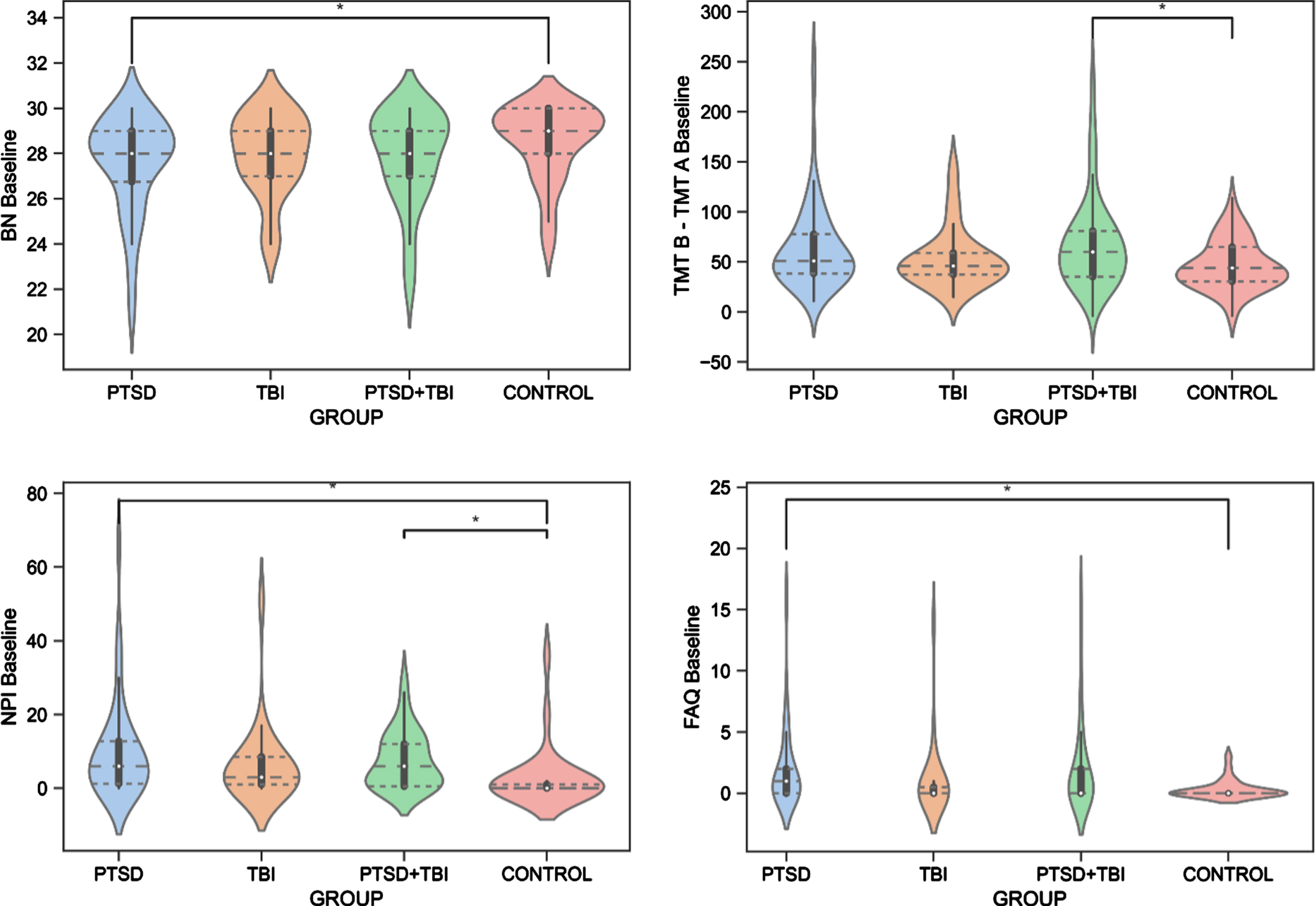
The ANCOVAs revealed significant differences between the groups for the BNT test (F(3, 278) = 3.046, p = 0.043, ηp2 = 0.040). Specifically, Veterans with PTSD had significantly worse (p = 0.020) confrontation naming abilities than Veteran controls. Moreover, the groups differed on the TMT B-A (F(3,275) = 3.125, p = 0.040, ηp2 = 0.033), revealing lower performance of executive functioning in Veterans with PTSD+TBI compared to Veteran controls (p = 0.040). No group effect was seen for the AVDEL30 (p > 0.05), measuring delayed memory performance. The Kruskal-Wallis test showed group differences in the NPI (H(3) = 17.888, [H] = 0.12, p = 0.001) and FAQ (H(3) = 9.965, [H] = 0.06, p = 0.020). Specifically, Veterans with PTSD and PTSD+TBI showed more neuropsychiatric disturbances than Veteran controls (p < 0.001), and Veterans with PTSD displayed higher FAQ scores than Veteran controls (p = 0.030), reflecting worse functioning in activities of daily living. The results are reported in Table 3 and Fig. 3, and detailed sample sizes are reported in Table 3.
Table 2
Sample characteristics at baseline
| PTSD | TBI | TBI+PTSD | Control | Total | p | ||
| n Mean±SD | n Mean±SD | n Mean±SD | n Mean±SD | n Mean±SD | p | ||
| Age (years) Education (years) Life CAPS | 78 68.10±3.32 14.54±2.36 74.25±18.02 | 43 70.37±5.42 16.05±2.30 14.56±9.69 | 93 69.83±2.96 14.84±2.48 59.70±18.87 | 71 71.40±5.79 16.06±2.13 5.95±8.15 | 285 69.84±4.49 15.24±2.41 44.20±32.50 | <0.001* <0.001* <0.001* | |
| % | % | % | % | % | p | ||
| Genotype | APOE ɛ4+ | 23.4% | 32.5% | 25.0% | 27.1% | 26.2% | 0.745 |
| Ethnicity | Hispanic | 12.8% | 4.7% | 6.5% | 5.6% | 7.7% | 0.476 |
| Caucasian | 85.9% | 93.0% | 92.5% | 94.4% | 91.2% | ||
| Unknown | 1.3% | 2.3% | 1.1% | 0% | 1.1% | ||
| Race | American Indian | 1.3% | 0% | 2.2% | 1.4% | 1.4% | 0.550 |
| Asian | 0% | 0% | 0% | 4.2% | 1.1% | ||
| African American | 7.7% | 9.3% | 8.6% | 5.6% | 7.7% | ||
| White | 84.6% | 86.0% | 81.7% | 85.9% | 84.2% | ||
| More than one race | 5.1% | 4.7% | 6.5% | 1.4% | 4.6% | ||
| Unknown | 1.3% | 0% | 1.1% | 1.4% | 1.1% | ||
| Language | English | 97.4% | 100% | 97.8% | 97.2% | 97.9% | 0.810 |
| Spanish | 2.6% | 0% | 1.1% | 1.4% | 1.4% | ||
| Other | 0% | 0% | 1.1% | 1.4% | 0.7% | ||
| Work Status | Working | 10.3% | 18.6% | 8.6% | 19.7% | 13.3% | 0.113 |
| Retired | 89.7% | 81.4% | 91.4% | 80.3% | 86.7% | ||
| Handedness | Right | 88.5% | 86.0% | 91.4% | 90.1% | 89.5% | 0.796 |
| Left | 11.5% | 14.0% | 8.6% | 9.9% | 10.5% | ||
| Marital Status | Married | 82.1% | 86.0% | 68.8% | 85.9% | 79.3% | 0.168 |
| Widowed | 3.8% | 0% | 8.6% | 4.2% | 4.9% | ||
| Divorced | 7.7% | 11.6% | 15.1% | 5.6% | 10.2% | ||
| Never married | 6.4% | 2.3% | 7.5% | 4.2% | 5.6% | ||
| Medication usage | No psychiatric med | 43.5% | 85.7% | 69.1% | 93.0% | 71.5% | <0.001* |
| Donezepil | 0% | 2.4% | 0% | 0% | 0.4% | ||
| Galantamine | 1.4% | 2.4% | 0% | 0% | 0.8% | ||
| Anti-depressants | 44.9% | 7.1% | 28.4% | 5.6% | 23.2% | ||
| Other psychiatric med | 10.1% | 2.4% | 2.5% | 1.4% | 4.2% |
CAPS, Clinician Administered PTSD Scale; GD, geriatric depression; med, medication; PTSD, posttraumatic stress disorder; SD, standard deviation; TBI, traumatic brain injury. *Significant differences in the one-way ANOVA (continuous variables) or Chi-square test (categorical variables).
Table 3
Cognitive, behavioral, and neuroimaging data at baseline
| Analyses Dependent variables | PTSD | TBI | PTSD+TBI | Control | TOTAL | p | FDR corrected p | Post hoc PTSD- TBI | Post hoc PTSD- Control | Post hoc PTSD- PTSD+ TBI | Post hoc TBI- PTSD+ TBI | Post hoc TBI- Control | Post hoc Control- PTSD+TBI | |||||
| ANCOVA | n | Mean±SD | n | Mean±SD | n | Mean±SD | n | Mean±SD | n | Mean±SD | p | p + | p + | p + | p + | p + | p + | p + |
| BNT | 78 | 27.50±2.12 | 42 | 28.05±1.70 | 93 | 27.82±2.11 | 71 | 28.54±1.66 | 284 | 27.94±1.98 | 0.03* | 0.04* | 1.00 | 0.02* | 1.00 | 1.00 | 0.99 | 0.27 |
| TMT B-A | 75 | 63.35±42.56 | 43 | 54.23±29.80 | 93 | 69.06±45.26 | 71 | 52.75±35.49 | 282 | 61.17±40.51 | 0.03* | 0.04* | 0.87 | 0.21 | 0.32 | 0.316 | 1.00 | 0.04* |
| AVDEL30 | 78 | 6.12±3.48 | 43 | 6.05±3.81 | 93 | 5.19±3.72 | 71 | 6.32±3.94 | 285 | 5.25±3.78 | 0.34 | 0.34 | ||||||
| Kruskal Wallis | n | Mean Rank | n | Mean Rank | n | Mean Rank | n | Mean Rank | n | p | p + | p + | p + | p + | p + | p + | p + | |
| NPI | 41 | 77.43 | 22 | 64.66 | 43 | 73.15 | 25 | 38.46 | 131 | <0.00* | 0.00* | 1.00 | 0.00* | 1.00 | 1.00 | 0.10 | 0.00* | |
| FAQ | 41 | 74.74 | 22 | 57.32 | 43 | 71.02 | 25 | 50.66 | 131 | 0.01* | 0.02* | 0.30 | 0.03* | 1.00 | 0.72 | 1.00 | 0.09 | |
| ANCOVA | n | Mean±SD | n | Mean±SD | n | Mean±SD | n | Mean±SD | n | Mean±SD | p | |||||||
| UF FA | 31 | 0.58±0.34 | 8 | .57±.05 | 23 | .57±.04 | 29 | .56±.04 | 91 | .56±.04 | .31 | |||||||
| CI FA | 30 | 0.51±0.48 | 8 | .55±.05 | 23 | .51±.04 | 26 | .53±.04 | 87 | .52±.05 | .22 | |||||||
| ILF FA | 29 | 0.67±0.04 | 10 | .68±.04 | 23 | .66±.04 | 33 | .66±.04 | 95 | .66±.04 | .36 | |||||||
| Florbetapir frontal1 | 70 | 1.21±.16 | 32 | 1.23±.26 | 61 | 1.28±.25 | 67 | 1.28±.23 | 230 | 1.25±.22 | .40 | |||||||
| Florbetapir cingulate1 | 70 | 1.34±.18 | 32 | 1.35±.27 | 61 | 1.41±.25 | 67 | 1.41±.25 | 230 | 1.38±.23 | .34 | |||||||
| Florbetapir parietal1 | 70 | 1.24±.14 | 32 | 1.26±.30 | 61 | 1.30±.23 | 67 | 1.30±.25 | 230 | 1.28±.23 | .60 | |||||||
| Florbetapir temporal1 | 70 | 1.15±.14 | 32 | 1.18±.25 | 61 | 1.21±.21 | 67 | 1.22±.22 | 230 | 1.19±.20 | .66 | |||||||
| Florbetapir composite cortical1 | 70 | 1.24±.15 | 32 | 1.26±.26 | 61 | 1.30±.23 | 67 | 1.30±.23 | 230 | 1.27±.22 | .57 | |||||||
| Flortaucipir temporal1 | 38 | 1.15±.09 | 18 | 1.18±.08 | 37 | 1.14±.12 | 27 | 1.15±.08 | 120 | 1.15±.10 | .68 | |||||||
AVDEL30, Auditory Verbal Learning Test 30 Minutes Delayed; BNT, Boston Naming Test; CI FA, cingulate fractional anisotropy; FAQ, Functional Assessment Questionnaire; FDR, false discovery rate; ILF FA, inferior longitudinal fasciculus fractional anisotropy; NPI, Neuropsychiatric Inventory; PTSD, posttraumatic stress disorder; SD, standard deviation; SUVR, standardized uptake value ratio; TBI, traumatic brain injury; TMT B-A, Trail Making Test B-A; UF FA, uncinate fasciculus fractional anisotropy.*Significant differences in the Kruskal-Wallis tests; p + FDR corrected; 1SUVR. Data for BNT, TMT B-A, AVDEL30 is reported from models correcting for age and education. Sample for the TMT B-A and BNT differs since three subjects miss the TMT B score and one the BNT score. Data for the neuroimaging measures from models correcting for age, education, and scanner id.
Fig. 3
Group Differences in Cognitive and Behavioral Functioning. BN, Boston Naming Test, Naming Abilities; FAQ, Functional Assessment Questionnaire, Functioning in Activities of Daily Living; NPI, Neuropsychiatric Inventory, Neuropsychiatric Disturbances; PTSD, posttraumatic stress disorder; TBI, traumatic brain injury; TMT B – TMT A, Trail Making Test B-A, Executive Functioning.
Group differences in neuroimaging measures
Differences in all sample characteristics among groups with and without available or analyzed DTI data were examined; groups did not differ in any demographic characteristics (p > 0.05), although participants without available or analyzed DTI data had higher lifetime CAPS, worse scores in the AVDEL30 test, had more PTSD+TBI and less control participants, and more participants with MCI diagnosis compared to participants with available or analyzed DTI data (results are reported in Supplementary Table 2). The ANCOVA analyses showed no significant differences in baseline FA for the three tracts (UF, CI, ILF) between Veterans with PTSD (n = 31), PTSD+TBI (n = 23), and Veteran controls (n = 29) [the group of Veterans with TBI only (n = 8) was not included due to the small sample size]. Moreover, no differences were shown in any of the florbetapir SUVR regions (frontal, cingulate, parietal, temporal, and composite cortical) nor in baseline flortaucipir temporal SUVR for all four groups (p > 0.05, Table 3).
Group differences in cognitive, behavioral, and neuroimaging score differences (follow-up – baseline)
ANCOVAs were performed to test for between-group differences in the cognitive and neuroimaging score differences from baseline to follow-up. After the removal of extreme cases, the time interval between the two assessments was of 1.07 (0.16) years for the cognitive and behavioral assessments ranging from 0.79 to 1.6 years; of 1.13 (0.12) for the diffusion MRI assessments ranging from to 1.02 to 1.44 years, and of 1.13 (0.22) ranging from 0.75 to 1.72 for the tau PET assessment. No significant group differences for score changes of the BNT, TMT B-A, AVDEL30, NPI, FAQ, and flortaucipir temporal SUVR were shown (p > 0.05).
There was a significant difference between Veterans with PTSD compared to Veteran controls in score changes (follow-up – baseline) of the UF FA (F(1,27)=10.27, p = 0.001,
DISCUSSION
The present study analyzed cognitive, behavioral, and neuroimaging data of a cohort of Vietnam war Veterans from the ADNI-DOD study. We showed that decades after trauma, 1) a diagnosis of PTSD or PTSD+TBI was associated with worse behavioral and cognitive functioning; 2) groups did not differ in white matter microstructure, Aβ and tau accumulation at baseline; 3) Veterans with PTSD showed a progressive decline in the UF microstructural integrity compared to Veteran controls over one year.
Baseline differences in cognitive and behavioral functioning
Consistent with our hypothesis, Veterans with PTSD+TBI showed worse executive functioning and Veterans with PTSD showed worse confrontation naming abilities compared to Veteran controls. It has been suggested that Veterans with PTSD may have decreased cognitive reserve—the ability to cope with the harmful effects of pathology on cognitive functioning [93]. Poorer cognitive performance and lower cognitive reserve may then increase the risk for a manifestation of cognitive impairments. The pre-dementia stage is characterized by deficits in various cognitive domains but intact daily function [94], and a study indicated decreased language abilities among the most discriminant indicator of cognitive decline [95]. Lower general cognition was also found in a study using the ADNI-DOD sample in the PTSD and PTSD+TBI groups compared to Veteran controls [59]. In addition to deficits in cognitive functioning, our study also showed worse daily functioning among Veterans with PTSD and more neuropsychiatric disturbances in both PTSD and PTSD+TBI compared to Veteran controls. These findings align with previous studies [96, 97], and impairments in behavioral functioning have also been linked to an increased risk of AD development [98–101]. Therefore, cognitive and behavioral functioning should be closely monitored in Veterans with PTSD.
Contrary to our hypothesis, Veterans with TBI did not differ from Veterans with PTSD, PTSD+TBI, or Veteran controls in cognitive or behavioral functioning. Moreover, no differences were found between Veterans with PTSD or TBI and those with PTSD+TBI. It can be argued that PTSD and the pathological sequelae of TBI may play a role in the pathogenesis of cognitive decline rather than being a necessary or sufficient condition to develop dementia [13]. Furthermore, in the present study, Veterans belonging to the four cohorts were exposed to substantial distress and likely under-reported TBI events during deployment, making it difficult to discriminate between groups.
Some of the differences among groups might have been evened out due to all subjects having had combat exposure. Additionally, a previous meta-analysis has reported that significant heterogeneity was present among studies comparing samples of Veterans with and without PTSD [102]. Despite this limitation, comparing four groups of Veterans with different diagnoses allows one to specifically study patterns of cognitive reserve and different vulnerability among combats.
No baseline differences in neuroimaging measures
In contrast to our hypothesis, we did not find significant differences in white matter measures, Aβ and tau at baseline between groups of Veterans with PTSD, TBI, and PTSD+TBI compared to Veteran controls. The null results for Aβ and tau suggest that the risk conferred by PTSD and TBI for cognitive decline might not be related to underlying AD pathology. While both PTSD [35, 36, 38, 39, 49, 103–116] and TBI [37, 44, 117–119] have repeatedly been linked to widespread abnormalities in white matter, some studies did not report significant differences between individuals with PTSD+TBI and controls [45, 120], consistent with our findings. Similarly, some studies reported differences in Aβ deposition [28, 70], while others, two of which also using ADNI-DOD data [25, 59], did not find any differences between Veterans with TBI [25, 29, 30, 121, 122] or PTSD [26] and Veteran controls. Our results on Aβ and tau are in accordance with a recent ADNI-DOD study that found no differences in Aβ and tau, as well as no differences in cerebrovascular disease measures [59]. Similarly, no differences were observed among individuals with a single moderate to severe TBI and controls in Aβ and tau burden years after the injury [123]. Analyzing these four groups separately allowed us to investigate whether PTSD or TBI as a loading factor to either condition led to a higher presence of AD biomarkers. This is particularly relevant since previous literature has shown that white matter abnormalities are exacerbated in PTSD+TBI [41, 124], also longitudinally [125]. It is possible that trauma-exposed Veteran controls present with an equally compromised brain structure as those who developed PTSD [126, 127]. Therefore, while our results are relevant for the Veteran population, future studies should investigate the impact of PTSD or TBI as loading factors in non-Veterans samples. Moreover, a TBI may lead to various presentations of brain abnormalities [6], which makes identifying a common pattern of imaging differences on a group level difficult. While it is beyond the scope of the current paper, considering mechanisms such as inflammation [128] or synaptic dysfunction [129] may be of value in future studies, given that AD onset comprises a cascade of different pathological mechanisms [130], not limited to Aβ deposition, tau accumulation, or white matter abnormalities.
Table 4
Cognitive, behavioral, and neuroimaging changes (Follow-up – Baseline)
| Analysis Dependent variables | PTSD | TBI | PTSD+TBI | Control | TOTAL | p | FDR corrected p | |||||
| ANCOVA Follow-up - baseline | N | Mean±SD | n | Mean±SD | n | Mean±SD | n | Mean±SD | n | Mean±SD | p | p + |
| BNT | 58 | 0.05±1.76 | 36 | 0.39±1.57 | 57 | 0.26±1.51 | 55 | 0.16±1.23 | 206 | 0.20±1.52 | 0.56 | 0.56 |
| TMT B-A | 55 | –0.67±33.83 | 36 | –5.23±26.61 | 57 | 8.05±47.50 | 55 | 7.81±35.11 | 203 | 3.22±37.60 | 0.13 | 0.20 |
| AVDEL30 | 58 | –0.40±3.01 | 36 | 0.86±3.51 | 57 | 0.65±3.42 | 55 | 1.01±3.18 | 206 | 0.49±3.29 | 0.12 | 0.20 |
| NPI | 27 | 0.30±11.00 | 16 | –0.50±6.00 | 25 | –2.40±5.76 | 21 | 0.14±9.77 | 89 | –0.46±8.61 | 0.58 | 0.58 |
| FAQ | 27 | –0.26±2.10 | 16 | –0.56±0.96 | 25 | –0.96±2.37 | 21 | 0.48±1.21 | 89 | –0.34±1.90 | 0.08 | 0.16 |
| UF FA | 17 | –0.02±0.03 | 4 | / | 4 | / | 15 | 0.01±0.02 | 40 | –0.00±0.03 | 0.00* | 0.00* |
| CI FA | 16 | –0.01±0.03 | 4 | / | 4 | / | 13 | –0.01±0.03 | 37 | 0.00±0.01 | 0.82 | 0.82 |
| ILF FA | 13 | –0.00±0.04 | 4 | / | 4 | / | 15 | 0.01±0.04 | 36 | 0.01±0.04 | 0.47 | 0.71 |
| Flortaucipir temporal1 | 19 | –0.01±0.05 | 10 | –0.03±0.03 | 20 | 0.01±0.04 | 13 | 0.01±0.03 | 62 | –0.00±0.04 | 0.12 | 0.12 |
AVDEL30, Auditory Verbal Learning Test 30 Minutes Delayed; BNT, Boston Naming Test; CI FA, cingulate fractional anisotropy; FAQ, Functional Assessment Questionnaire; ILF FA, inferior longitudinal fasciculus fractional anisotropy; NPI, Neuropsychiatric Inventory; PTSD, posttraumatic stress disorder; SD, standard deviation; TBI, traumatic brain injury; TMT B-A, Trail Making Test B-A; UF FA, uncinate fasciculus fractional anisotropy.*Significant differences in the ANCOVA. p + FDR corrected. 1SUVR. Data for BNT, TMT B-A, AVDEL30, NPI, FAQ is reported from models correcting for age and education; data from FA corrected for age, education, and scanner id. Sample for the TMT B-A differs since three subjects miss TMT B at baseline. No differences were calculated for amyloid PET since insufficient follow-up data was available, due to budget restrictions as of protocol.
White matter microstructure change in PTSD compared to controls
Monitoring longitudinal changes is critical when assessing the impact of PTSD and TBI on cognitive decline. We compared the differences in cognitive and behavioral functioning and white matter change from baseline to one-year follow-up between groups. While Veterans with PTSD and PTSD+TBI showed worsening in some of the cognitive and behavioral functioning domains, the groups did not differ significantly from each other, Veterans with TBI, or Veteran controls. Therefore, the findings suggest no accelerated decline of cognitive and behavioral functioning in Veterans diagnosed with PTSD, TBI, or PTSD+TBI for the studied age range and timespan.
Fig.4
UF FA Change Difference Between Veterans with PTSD and Veteran Controls. PTSD, posttraumatic stress disorder; UF, uncinate fasciculus; FA, fractional anisotropy.
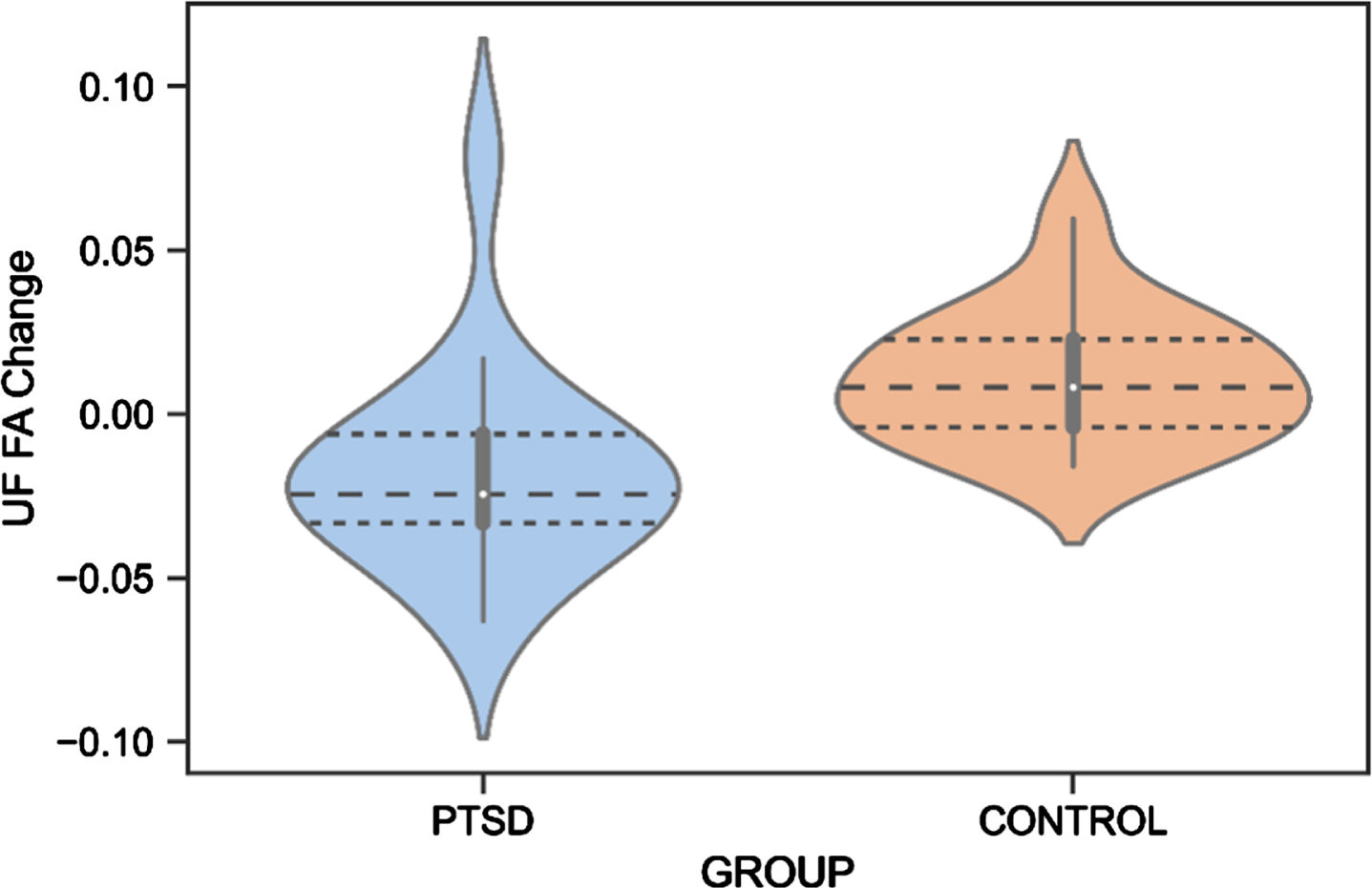
Regarding neuroimaging measures, white matter changes have been suggested as a biomarker for the early identification of AD [131], and alterations in the UF, CI, and ILF white matter microstructure have previously been found in AD patients [131–133]. We reported that white matter microstructure of the UF decreased in Veterans with PTSD, while an improvement was found for the control group. Although an increase in white matter microstructure is not expected at an older age, Veteran controls may still be socially and cognitively active, potentially leading to neural restructuring. Due to the small sample size in these analyses, these preliminary findings should be interpreted cautiously. Previous studies reported abnormalities of the UF in individuals with PTSD [43, 113], although no study has yet investigated this longitudinally. Since the UF is involved in emotion processing, memory, and language functions [81], it is highly responsible for cognitive features commonly impacted in AD [134]. For example, a decrease in the number of fibers in the right UF has been associated with memory loss in individuals with mild cognitive impairments (MCI) [135]. Moreover, a relationship between decreased UF FA and poor performance on the Mini-Mental State Examination, a test for cognitive functioning in the elderly, was shown in individuals with probable AD [136]. Increased risk of dementia with considerably low FA of the UF was also found in individuals with amnestic MCI [137] and semantic dementia [138]. Our preliminary finding of a decrease in UF FA in Veterans with PTSD is thus in line with previous research and may indicate an early cognitive decline. Importantly, the UF finding might not be specific to AD but related to other neurodegenerative pathologies or to aging in general, considering that previous literature has found abnormalities in the UF also in frontotemporal dementia [139, 140].
Previous studies have found an association between cognitive reserve proxies and white matter integrity, supporting the involvement of white matter changes in processes of cognitive reserve. Cognitive reserve might also affect white matter microstructural changes over time, and eventually predispose veterans with PTSD to develop cognitive impairment [141, 142]. In a recent study, associations between cognitive reserve and white matter microstructure measured over time were found to differ by age in healthy older adults, suggesting that cognitive reserve has a neuroprotective role in middle age and shifts to a compensatory effect in older age [143].
No significant changes were observed from baseline to after one year in ILF and CI microstructure between Veterans with PTSD and Veteran controls. In a recent study involving individuals with MCI, the ILF was one of the tracts indicating the risk of conversion to AD [144]. In the current study, the ILF microstructure slightly decreased in the PTSD group and increased in the Veteran control group without reaching statistical significance, while a decrease in CI microstructure was seen in Veterans with PTSD and Veteran controls. Future research should investigate these trends using larger sample sizes.
Limitations and future directions
We acknowledge several study limitations. Group differences in white matter microstructural changes of the TBI and PTSD+TBI groups were not investigated, given the small sample sizes. The same applied to follow-up amyloid-PET measures. In addition, we did not control for exposures to psychological or brain trauma or treatment during the follow-up year. Furthermore, additional information on the trauma (i.e., time since trauma, severity, number of TBIs) would have been beneficial for interpreting the findings. An additional limitation is that results might have varied depending on MCI diagnostic status. Although, we were unable to perform these subgroup analyses due to insufficient sample size within each PTSD and/or TBI group. However, removing MCI subjects would have led to biases in the selection of the sample, since individuals with TBI and PTSD might have a greater incidence of cognitive impairment. A major limitation of the present study is the small sample size for the white matter longitudinal analysis, calling for future studies validating these preliminary findings with a larger sample. Nevertheless, the current study provides meaningful insights into the longitudinal effects of PTSD and TBI on cognitive and behavioral functioning and brain structure that may indicate AD development.
Additional follow-up analyses are needed to validate the result of progressive white matter decline in Veterans with PTSD. This would allow conclusions on trajectories that cannot be reliably derived from only two assessment time points. Studies repeating assessments with a longer follow-up time are needed to assess whether progressive changes in cognition are seen after a longer period than one year. Although, considering the mean age of our sample, changes in AD markers can be expected after one year. Furthermore, accompanying Veterans with interventions to alleviate PTSD and TBI-related symptoms would be highly meaningful when studying the potential preventive effects of stress reduction and cognitive training for dementia onset. Moreover, a healthy control group of non-Veterans is needed to validate our findings. Lastly, future studies should consider assessing resilience predisposing factors (e.g., perceived health, sex, trait self-enhancement [145]) and investigate whether these influence the advent of cognitive decline in the Veteran population.
Conclusion
PTSD and PTSD+TBI negatively impact behavioral and cognitive functioning in the aging Veteran population. Moreover, our findings suggest a decrease in UF white matter microstructure in Veterans with PTSD after one year. Differences in baseline neuroimaging measures and behavioral and cognitive change were not identified. Therefore, while PTSD and PTSD+TBI confer risk for cognitive decline, our results do not support a direct link with AD pathology given a lack of difference in episodic memory, Aβ, and tau. Instead, longitudinal white matter changes might account for the decline, but this preliminary result should be further investigated. We conclude that Veterans with PTSD and PTSD+TBI need to be monitored and treated adequately to prevent behavioral and cognitive decline.
ACKNOWLEDGMENTS
Contribution was given by Zhenya Knyazhanskaya who worked on image preprocessing and manual ROI delineation reliability (together with Brynn Vessey for the latter).
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (http://www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
FUNDING
JSH receives funding for a Fellowship Award from Harvard Medical School Livingston and for a Young Investigator Grant sponsored by Mary and John Osterhaus and the Brain & Behavior Research Foundation.
CONFLICT OF INTEREST
Michael L. Alosco is an Editorial Board Member of this journal but was not involved in the peer-review process nor had access to any information regarding its peer-review.
All other authors have no conflict of interest to report.
DATA AVAILABILITY
Data used to prepare this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (http://adni.loni.usc.edu).
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-221304.
REFERENCES
[1] | Epidemiology of PTSD, https://mirecc.va.gov/mirecc/cih-visn2/Documents/Provider_Education_Handouts/Epidemiology_of_PTSD_Version_3.pdf. |
[2] | VA research on Traumatic Brain Injury (TBI), https://www.research.va.gov/topics/tbi.cfm. |
[3] | Stricker NH , Lippa SM , Green DL , McGlynn SM , Grande LJ , Milberg WP , McGlinchey RE ((2017) ) Elevated rates of memory impairment in military service-members and veterans with posttraumatic stress disorder. J Clin Exp Neuropsychol 39: , 768–785. |
[4] | Griesbach GS , Masel BE , Helvie RE , Ashley MJ ((2018) ) The impact of traumatic brain injury on later life: Effects on normal aging and neurodegenerative diseases. J Neurotrauma 35: , 17–24. |
[5] | van der Horn HJ , Out ML , de Koning ME , Mayer AR , Spikman JM , Sommer IE , van der Naalt J ((2020) ) An integrated perspective linking physiological and psychological consequences of mild traumatic brain injury. J Neurol 267: , 2497–2506. |
[6] | Pavlovic D , Pekic S , Stojanovic M , Popovic V ((2019) ) Traumatic brain injury: Neuropathological, neurocognitive and neurobehavioral sequelae. Pituitary 22: , 270–282. |
[7] | Brenner LA ((2011) ) Neuropsychological and neuroimaging findings in traumatic brain injury and post-traumatic stress disorder. Dialogues Clin Neurosci 13: , 311–323. |
[8] | Kaplan GB , Leite-Morris KA , Wang L , Rumbika KK , Heinrichs SC , Zeng X , Wu L , Arena DT , Teng YD ((2018) ) Pathophysiological bases of comorbidity: Traumatic brain injury and post-traumatic stress disorder. J Neurotrauma 35: , 210–225. |
[9] | Rabinowitz AR , Watanabe TK ((2020) ) Pharmacotherapy for treatment of cognitive and neuropsychiatric symptoms after mTBI. J Head Trauma Rehabil 35: , 76–83. |
[10] | Livingston G , Huntley J , Sommerlad A , Ames D , Ballard C , Banerjee S , Brayne C , Burns A , Cohen-Mansfield J , Cooper C , Costafreda SG , Dias A , Fox N , Gitlin LN , Howard R , Kales HC , Kivimäki M , Larson EB , Ogunniyi A , Orgeta V , Ritchie K , Rockwood K , Sampson EL , Samus Q , Schneider LS , Selbæk G , Teri L , Mukadam N ((2020) ) Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396: , 413–446. |
[11] | Barnes DE , Kaup A , Kirby KA , Byers AL , Diaz-Arrastia R , Yaffe K ((2014) ) Traumatic brain injury and risk of dementia in older veterans. Neurology 83: , 312–319. |
[12] | Barnes DE , Byers AL , Gardner RC , Seal KH , Boscardin WJ , Yaffe K ((2018) ) Association of mild traumatic brain injury with and without loss of consciousness with dementia in US military veterans. JAMA Neurol 75: , 1055–1061. |
[13] | Greenberg MS , Tanev K , Marin M , Pitman RK ((2014) ) Stress, PTSD, and dementia. Alzheimers Dement 10: , S155–S165. |
[14] | Weiner MW , Friedl KE , Pacifico A , Chapman JC , Jaffee MS , Little DM , Manley GT , McKee A , Petersen RC , Pitman RK , Yaffe K , Zetterberg H , Obana R , Bain LJ , Carrillo MC ((2013) ) Military risk factors for Alzheimer’s disease. Alzheimers Dement 9: , 445–451. |
[15] | Braak H , Braak E ((1991) ) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82: , 239–259. |
[16] | Mesulam MM ((1999) ) Neuroplasticity failure in Alzheimer’s disease: Bridging the gap between plaques and tangles. Neuron 24: , 521–529. |
[17] | Jack CR Jr , Bennett DA , Blennow K , Carrillo MC , Dunn B , Haeberlein SB , Holtzman DM , Jagust W , Jessen F , Karlawish J , Liu E , Molinuevo JL , Montine T , Phelps C , Rankin KP , Rowe CC , Scheltens P , Siemers E , Snyder HM , Sperling R; Contributors ((2018) ) NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14: , 535–562. |
[18] | Clark CM , Schneider JA , Bedell BJ , Beach TG , Bilker WB , Mintun MA , Pontecorvo MJ , Hefti F , Carpenter AP , Flitter ML , Krautkramer MJ , Kung HF , Coleman RE , Doraiswamy PM , Fleisher AS , Sabbagh MN , Sadowsky CH , Reiman EP , Zehntner SP , Skovronsky DM; AV45-A07 Study Group ((2011) ) Use of florbetapir-PET for imaging beta-amyloid pathology. JAMA 305: , 275–283. |
[19] | Jeong YH , Park CH , Yoo J , Shin KY , Ahn SM , Kim HS , Lee SH , Emson PC , Suh YH ((2006) ) Chronic stress accelerates learning and memory impairments and increases amyloid deposition in APPV717I-CT100 transgenic mice, an Alzheimer’s disease model. FASEB J 20: , 729–731. |
[20] | Catania C , Sotiropoulos I , Silva R , Onofri C , Breen KC , Sousa N , Almeida OFX ((2009) ) The amyloidogenic potential and behavioral correlates of stress. Mol Psychiatry 14: , 95–105. |
[21] | Lucassen PJ , Pruessner J , Sousa N , Almeida OFX , van Dam AM , Rajkowska G , Swaab DF , Czéh B ((2014) ) Neuropathology of stress. Acta Neuropathol 127: , 109–135. |
[22] | Justice NJ , Huang L , Tian JB , Cole A , Pruski M , Hunt AJ , Flores R , Zhu MX , Arenkiel BR , Zheng H ((2015) ) Posttraumatic stress disorder-like induction elevates β-amyloid levels, which directly activates corticotropin-releasing factor neurons to exacerbate stress responses. J Neurosci 35: , 2612–2623. |
[23] | Elias A , Rowe C , Hopwood M ((2021) ) Risk of dementia in posttraumatic stress disorder. J Geriatr Psychiatry Neurol 34: , 555–564. |
[24] | Clouston SAP , Deri Y , Diminich E , Kew R , Kotov R , Stewart C , Yang X , Gandy S , Sano M , Bromet EJ , Luft BJ ((2019) ) Posttraumatic stress disorder and total amyloid burden and amyloid-β 42/40 ratios in plasma: Results from a pilot study of World Trade Center responders. Alzheimers Dement (Amst) 11: , 216–220. |
[25] | Weiner MW , Harvey D , Hayes J , Landau SM , Aisen PS , Petersen RC , Tosun D , Veitch DP , Jack CR Jr , Decarli C , Saykin AJ , Grafman J , Neylan TC; Department of Defense Alzheimer’s Disease Neuroimaging Initiative ((2017) ) Effects of traumatic brain injury and posttraumatic stress disorder on development of Alzheimer’s disease in Vietnam Veterans using the Alzheimer’s Disease Neuroimaging Initiative: Preliminary report. Alzheimers Dement (N Y) 3: , 177–188. |
[26] | Elias A , Cummins T , Lamb F , Tyrrell R , Dore V , Williams R , Rosenfeld JV , Hopwood M , Villemagne VL , Rowe CC ((2020) ) Amyloid-β, tau, and 18F-fluorodeoxyglucose positron emission tomography in posttraumatic stress disorder. J Alzheimers Dis 73: , 163–173. |
[27] | Johnson VE , Stewart W , Smith DH ((2012) ) Widespread tau and amyloid-beta pathology many years after a single traumatic brain injury in humans. Brain Pathol 22: , 142–149. |
[28] | Scott G , Ramlackhansingh AF , Edison P , Hellyer P , Cole J , Veronese M , Leech R , Greenwood RJ , Turkheimer FE , Gentleman SM , Heckemann RA , Matthews PM , Brooks DJ , Sharp DJ ((2016) ) Amyloid pathology and axonal injury after brain trauma. Neurology 86: , 821–828. |
[29] | Crane PK , Gibbons LE , Dams-O’Connor K , Trittschuh E , Leverenz JB , Dirk Keene C , Sonnen J , Montine TJ , Bennett DA , Leurgans S , Schneider JA , Larson EB ((2016) ) Association of traumatic brain injury with late-life neurodegenerative conditions and Neuropathologic findings. JAMA Neurol 73: , 1062–1069. |
[30] | Asken BM , Mantyh WG , la Joie R , Strom A , Casaletto KB , Staffaroni AM , Apple AC , Lindbergh CA , Iaccarino L , You M , Grant H , Fonseca C , Windon C , Younes K , Tanner J , Rabinovici GD , Kramer JH , Gardner RC ((2021) ) Association of remote mild traumatic brain injury with cortical amyloid burden in clinically normal older adults. Brain Imaging Behav 15: , 2417–2425. |
[31] | Araque Caballero MÁ , Suárez-Calvet M , Duering M , Franzmeier N , Benzinger T , Fagan AM , Bateman RJ , Jack CR , Levin J , Dichgans M , Jucker M , Karch C , Masters CL , Morris JC , Weiner M , Rossor M , Fox NC , Lee JH , Salloway S , Danek A , Goate A , Yakushev I , Hassenstab J , Schofield PR , Haass C , Ewers M ((2018) ) White matter diffusion alterations precede symptom onset in autosomal dominant Alzheimer’s disease. Brain 141: , 3065–3080. |
[32] | Pietroboni AM , Colombi A , Carandini T , Scarpini E , Galimberti D , Bozzali M ((2020) ) The role of amyloid-β in white matter damage: Possible common pathogenetic mechanisms in neurodegenerative and demyelinating diseases. J Alzheimers Dis 78: , 13–22. |
[33] | Moody JF , Dean DC , Kecskemeti SR , Blennow K , Zetterberg H , Kollmorgen G , Suridjan I , Wild N , Carlsson CM , Johnson SC , Alexander AL , Bendlin BB ((2022) ) Associations between diffusion MRI microstructure and cerebrospinal fluid markers of Alzheimer’s disease pathology and neurodegeneration along the Alzheimer’s disease continuum. Alzheimers Dement (Amst) 14: , e12381. |
[34] | Davenport ND , Lim KO , Sponheim SR ((2015) ) White matter abnormalities associated with military PTSD in the context of blast TBI. Hum Brain Mapp 36: , 1053–1064. |
[35] | Schuff N , Zhang Y , Zhan W , Lenoci M , Ching C , Boreta L , Mueller SG , Wang Z , Marmar CR , Weiner MW , Neylan TC ((2011) ) Patterns of altered cortical perfusion and diminished subcortical integrity in posttraumatic stress disorder: An MRI study. Neuroimage 54: Suppl 1, S62–S68. |
[36] | Aschbacher K , Mellon SH , Wolkowitz OM , Henn-Haase C , Yehuda R , Flory JD , Bierer LM , Abu-Amara D , Marmar CR , Mueller SG ((2018) ) Posttraumatic stress disorder, symptoms, and white matter abnormalities among combat-exposed veterans. Brain Imaging Behav 12: , 989–999. |
[37] | Rutgers DR , Fillard P , Paradot G , Tadié M , Lasjaunias P , Ducreux D ((2008) ) Diffusion tensor imaging characteristics of the corpus callosum in mild, moderate, and severe traumatic brain injury. Am J Neuroradiol 29: , 1730–1735. |
[38] | Bierer LM , Ivanov I , Carpenter DM , Wong EW , Golier JA , Tang CY , Yehuda R ((2015) ) White matter abnormalities in Gulf War veterans with posttraumatic stress disorder: A pilot study. Psychoneuroendocrinology 51: , 567–576. |
[39] | Sanjuan PM , Thoma R , Claus ED , Mays N , Caprihan A ((2013) ) Reduced white matter integrity in the cingulum and anterior corona radiata in posttraumatic stress disorder in male combat veterans: A diffusion tensor imaging study. Psychiatry Res Neuroimaging 214: , 260–268. |
[40] | Davenport ND , Lamberty GJ , Nelson NW , Lim KO , Armstrong MT , Sponheim SR ((2016) ) PTSD confounds detection of compromised cerebral white matter integrity in military veterans reporting a history of mild traumatic brain injury. Brain Inj 30: , 1491–1500. |
[41] | Lepage C , Pasternak O , Bouix S , Shenton ME , Zafonte RD , Coleman MJ , McAllister TW , Flashman LA , George MS , Morey RA , Marx CE , Coimbra R , Stein MB , Grant G , Andaluz N , de Pierrefeu A , Koerte IK , Shutter L ((2017) ) White matter abnormalities in mild traumatic brain injury with and without post-traumatic stress disorder: A subject-specific diffusion tensor imaging study. Brain Imaging Behav 12: , 870–881. |
[42] | Lopez KC , Leary JB , Pham DL , Chou YY , Dsurney J , Chan L ((2017) ) Brain volume, connectivity, and neuropsychological performance in mild traumatic brain injury: The impact of post-traumatic stress disorder symptoms. J Neurotrauma 34: , 16–22. |
[43] | Santhanam P , Teslovich T , Wilson SH , Yeh PH , Oakes TR , Weaver LK ((2019) ) Decreases in white matter integrity of ventro-limbic pathway linked to post-traumatic stress disorder in mild traumatic brain injury. J Neurotrauma 36: , 1093–1098. |
[44] | Petrie EC , Cross DJ , Yarnykh VL , Richards T , Martin NM , Pagulayan K , Hoff D , Hart K , Mayer C , Tarabochia M , Raskind MA , Minoshima S , Peskind ER ((2014) ) Neuroimaging, behavioral, and psychological sequelae of repetitive combined blast/impact mild traumatic brain injury in Iraq and Afghanistan war veterans. J Neurotrauma 31: , 425–436. |
[45] | Lange RT , Lippa SM , Brickell TA , Yeh P-H , Ollinger J , Wright M , Driscoll A , Sullivan J , Braatz S , Gartner R , Barnhart E , French LM ((2021) ) Post-traumatic stress disorder is associated with neuropsychological outcome but not white matter integrity after mild traumatic brain injury. J Neurotrauma 38: , 63–73. |
[46] | Zhang X , Sun Y , Li W , Liu B , Wu W , Zhao H , Liu R , Zhang Y , Yin Z , Yu T , Qing Z , Zhu B , Xu Y , Nedelska Z , Hort J , Zhang B ((2019) ) Characterization of white matter changes along fibers by automated fiber quantification in the early stages of Alzheimer’s disease. Neuroimage Clin 22: , 101723. |
[47] | Wen Q , Mustafi SM , Li J , Risacher SL , Tallman E , Brown SA , West JD , Harezlak J , Farlow MR , Unverzagt FW , Gao S , Apostolova LG , Saykin AJ , Wu YC ((2019) ) White matter alterations in early-stage Alzheimer’s disease: A tract-specific study. Alzheimers Dement (Amst) 11: , 576–587. |
[48] | lo Buono V , Palmeri R , Corallo F , Allone C , Pria D , Bramanti P , Marino S ((2020) ) Diffusion tensor imaging of white matter degeneration in early stage of Alzheimer’s disease: A review. Int J Neurosci 130: , 243–250. |
[49] | Dennis EL , Disner SG , Fani N , Salminen LE , Logue M , Clarke EK , Haswell CC , Averill CL , Baugh LA , Bomyea J , Bruce SE , Cha J , Choi K , Davenport ND , Densmore M , du Plessis S , Forster GL , Frijling JL , Gonenc A , Gruber S , Grupe DW , Guenette JP , Hayes J , Hofmann D , Ipser J , Jovanovic T , Kelly S , Kennis M , Kinzel P , Koch SBJ , Koerte I , Koopowitz S , Korgaonkar M , Krystal J , Lebois LAM , Li G , Magnotta VA , Manthey A , May GJ , Menefee DS , Nawijn L , Nelson SM , Neufeld RWJ , Nitschke JB , O’Doherty D , Peverill M , Ressler KJ , Roos A , Sheridan MA , Sierk A , Simmons A , Simons RM , Simons JS , Stevens J , Suarez-Jimenez B , Sullivan DR , Théberge J , Tran JK , van den Heuvel L , van der Werff SJA , van Rooij SJH , van Zuiden M , Velez C , Verfaellie M , Vermeiren RRJM , Wade BSC , Wager T , Walter H , Winternitz S , Wolff J , York G , Zhu Y , Zhu X , Abdallah CG , Bryant R , Daniels JK , Davidson RJ , Fercho KA , Franz C , Geuze E , Gordon EM , Kaufman ML , Kremen WS , Lagopoulos J , Lanius RA , Lyons MJ , McCauley SR , McGlinchey R , McLaughlin KA , Milberg W , Neria Y , Olff M , Seedat S , Shenton M , Sponheim SR , Stein DJ , Stein MB , Straube T , Tate DF , van der Wee NJA , Veltman DJ , Wang L , Wilde EA , Thompson PM , Kochunov P , Jahanshad N , Morey RA ((2021) ) Altered white matter microstructural organization in posttraumatic stress disorder across 3047 adults: Results from the PGC-ENIGMA PTSD consortium. Mol Psychiatry 26: , 4315–4330. |
[50] | Zhang Y , Chen H , Qi R , Ke J , Xu Q , Zhong Y , Wu Y , Guo Y , Lu G , Chen F ((2023) ) Aberrant white matter microstructure evaluation by automated fiber quantification in typhoon-related post-traumatic stress disorder. Brain Imaging Behav 17: , 213–222. |
[51] | Zemmoura I , Burkhardt E , Herbet G ((2021) ) The inferior longitudinal fasciculus: Anatomy, function and surgical considerations. J Neurosurg Sci 65: , 590–604. |
[52] | Papagno C , Miracapillo C , Casarotti A , Romero Lauro LJ , Castellano A , Falini A , Casaceli G , Fava E , Bello L ((2011) ) What is the role of the uncinate fasciculus? Surgical removal and proper name retrieval. Brain 134: , 405–414. |
[53] | Bubb EJ , Metzler-Baddeley C , Aggleton JP ((2018) ) The cingulum bundle: Anatomy, function, and dysfunction. Neurosci Biobehav Rev 92: , 104–127. |
[54] | Sugarman MA , McKee AC , Stein TD , Tripodis Y , Besser LM , Martin B , Palmisano JN , Steinberg EG , O’Connor MK , Au R , McClean M , Killiany R , Mez J , Weiner MW , Kowall NW , Stern RA , Alosco ML ((2019) ) Failure to detect an association between self-reported traumatic brain injury and Alzheimer’s disease neuropathology and dementia. Alzheimers Dement 15: , 686–698. |
[55] | Kuring JK , Mathias JL , Ward L ((2020) ) Risk of dementia in persons who have previously experienced clinically-significant depression, anxiety, or PTSD: A systematic review and meta-analysis. J Affect Disord 274: , 247–261. |
[56] | Gu D , Ou S , Liu G ((2022) ) Traumatic brain injury and risk of dementia and Alzheimer’s disease: A systematic review and meta-analysis. Neuroepidemiology 56: , 4–16. |
[57] | Graham A , Livingston G , Purnell L , Huntley J ((2022) ) Mild traumatic brain injuries and future risk of developing Alzheimer’s disease: Systematic review and meta-analysis. J Alzheimers Dis 87: , 969–979. |
[58] | Weiner MW , Veitch DP , Hayes J , Neylan T , Grafman J , Aisen PS , Petersen RC , Jack C , Jagust W , Trojanowski JQ , Shaw LM , Saykin AJ , Green RC , Harvey D , Toga AW , Friedl KE , Pacifico A , Sheline Y , Yaffe K , Mohlenoff B; Department of Defense Alzheimer’s Disease Neuroimaging Initiative ((2014) ) Effects of traumatic brain injury and posttraumatic stress disorder on Alzheimer’s disease in veterans, using the Alzheimer’s Disease Neuroimaging Initiative. Alzheimers Dement 10: , S226–S235. |
[59] | Weiner MW , Harvey D , Landau SM , Veitch DP , Neylan TC , Grafman JH , Aisen PS , Petersen RC , Jack CR Jr , Tosun D , Shaw LM , Trojanowski JQ , Saykin AJ , Hayes J , De Carli C ((2023) ) Traumatic brain injury and post-traumatic stress disorder are not associated with Alzheimer’s disease pathology measured with biomarkers. Alzheimers Dement 19: , 884–895. |
[60] | Mohamed AZ , Cumming P , Götz J , Nasrallah F ((2019) ) Tauopathy in veterans with long-term posttraumatic stress disorder and traumatic brain injury. Eur J Nucl Med Mol Imaging 46: , 1139–1151. |
[61] | Mohamed AZ , Cumming P , Nasrallah FA ((2021) ) White matter alterations are associated with cognitive dysfunction decades after moderate-to-severe traumatic brain injury and/or posttraumatic stress disorder. Biol Psychiatry Cogn Neurosci Neuroimaging 6: , 1100–1109. |
[62] | Effects of traumatic brain injury and post traumatic stress disorder on Alzheimer’s disease (AD) in Veterans using ADNI (DoD-ADNI) Psychological Evaluation/PTSD Core Traumatic Brain Injury/TBI Core, https://adni.loni.usc.edu/wp-content/uploads/2013/09/DOD-ADNI-IRB-Approved-Final-protocol-08072012.pdf. |
[63] | Cetin Karayumak S , Bouix S , Ning L , James A , Crow T , Shenton M , Kubicki M , Rathi Y ((2019) ) Retrospective harmonization of multi-site diffusion MRI data acquired with different acquisition parameters. Neuroimage 184: , 180–200. |
[64] | Ning L , Bonet-Carne E , Grussu F , Sepehrband F , Kaden E , Veraart J , Blumberg SB , Khoo CS , Palombo M , Kokkinos I , Alexander DC , Coll-Font J , Scherrer B , Warfield SK , Karayumak SC , Rathi Y , Koppers S , Weninger L , Ebert J , Merhof D , Moyer D , Pietsch M , Christiaens D , Gomes Teixeira RA , Tournier JD , Schilling KG , Huo Y , Nath V , Hansen C , Blaber J , Landman BA , Zhylka A , Pluim JPW , Parker G , Rudrapatna U , Evans J , Charron C , Jones DK , Tax CMW ((2020) ) Cross-scanner and cross-protocol multi-shell diffusion MRI data harmonization: Algorithms and results. Neuroimage 221: , 117128. |
[65] | Williams BW , Mack W , Henderson VW ((1989) ) Boston naming test in Alzheimer’s disease. Neuropsychologia 27: , 1073–1079. |
[66] | Reitan RM ((1958) ) Validity of the trail maiking test as an indicator of organic brain damage. Percept Mot Skills 8: , 271–276. |
[67] | Schmidt M ((1996) ) Rey Auditory verbal learning test: A handbook. Western Psychological Services. |
[68] | Cummings JL ((1997) ) The Neuropsychiatric Inventory: Assessing psychopathology in dementia patients. Neurology 48: , S10–S16. |
[69] | Pfeffer RI , Kurosaki TT , Harrah CHJ , Chance JM , Filos S ((1982) ) Measurement of functional activities in older adults in the community. J Gerontol 37: , 323–329. |
[70] | Mohamed AZ , Cumming P , Srour H , Gunasena T , Uchida A , Haller CN , Nasrallah F ((2018) ) Amyloid pathology fingerprint differentiates post-traumatic stress disorder and traumatic brain injury. Neuroimage Clin 19: , 716–726. |
[71] | Flortaucipir (AV-1451) processing methods, https://adni.bitbucket.io/reference/docs/UCBERKELEYAV1451/UCBERKELEY_AV1451_Methods_2021-01-14.pdf. |
[72] | DOD ADNI procedures manual, https://adni.loni.usc.edu/wp-content/uploads/2017/09/DODADNI_Procedures_Manual_20170912.pdf. |
[73] | reckbo, Tashrif Billah, Isaiah Norton (2019) pnlbwh/pnlpipe: Easy install and multiprocessing. |
[74] | Fedorov A , Beichel R , Kalpathy-Cramer J , Finet J , Fillion-Robin JC , Pujol S , Bauer C , Jennings D , Fennessy F , Sonka M , Buatti J , Aylward S , Miller JV , Pieper S , Kikinis R ((2012) ) 3D slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging 30: , 1323–1341. |
[75] | Malcolm JG , Shenton ME , Rathi Y ((2009) ) Neural tractography using an unscented Kalman filter. Inf Process Med Imaging 21: , 126–138. |
[76] | Malcolm JG , Shenton ME , Rathi Y ((2010) ) Filtered multitensor tractography. IEEE Trans Med Imaging 29: , 1664–1675. |
[77] | Baumgartner C , Michailovich O , Levitt J , Pasternak O , Bouix S , Westin CF , Rathi Y ((2012) ) A unified tractography framework for comparing diffusion models on clinical scans.. International Conference on Medical Image Computing and Computer Assisted Intervention (MICCAI 12) - Computational Diffusion MRI Workshop. |
[78] | Pasternak O , Sochen N , Gur Y , Intrator N , Assaf Y ((2009) ) Free water elimination and mapping from diffusion MRI. Magn Reson Med 62: , 717–730. |
[79] | Zhang F , Cetin Karayumak S , Hoffmann N , Rathi Y , Golby AJ , O’Donnell LJ ((2020) ) Deep white matter analysis (DeepWMA): Fast and consistent tractography segmentation. Med Image Anal 65: , 101761. |
[80] | Chen Z , Tie Y , Olubiyi O , Rigolo L , Mehrtash A , Norton I , Pasternak O , Rathi Y , Golby AJ , O’Donnell LJ ((2015) ) Reconstruction of the arcuate fasciculus for surgical planning in the setting of peritumoral edema using two-tensor unscented Kalman filter tractography. Neuroimage Clin 7: , 815–822. |
[81] | Catani M , Thiebaut de Schotten M ((2008) ) A diffusion tensor imaging tractography atlas for virtualdissections. Cortex 44: , 1105–1132. |
[82] | Sydnor VJ , Rivas-Grajales AM , Lyall AE , Zhang F , Bouix S , Karmacharya S , Shenton ME , Westin CF , Makris N , Wassermann D , O’Donnell LJ , Kubicki M ((2018) ) A comparison of three fiber tract delineation methods and their impact on white matter analysis. Neuroimage 178: , 318–331. |
[83] | Squarcina L , Bertoldo A , Ham TE , Heckemann R , Sharp DJ ((2012) ) A robust method for investigating thalamic white matter tracts after traumatic brain injury. Neuroimage 63: , 779–788. |
[84] | Psomiades M , Fonteneau C , Mondino M , Luck D , Haesebaert F , Suaud-Chagny MF , Brunelin J ((2016) ) Integrity of the arcuate fasciculus in patients with schizophrenia with auditory verbal hallucinations: A DTI-tractography study. Neuroimage Clin 12: , 970–975. |
[85] | Seitz J , Zuo JX , Lyall AE , Makris N , Kikinis Z , Bouix S , Pasternak O , Fredman E , Duskin J , Goldstein JM , Petryshen TL , Mesholam-Gately RI , Wojcik J , McCarley RW , Seidman LJ , Shenton ME , Koerte IK , Kubicki M ((2016) ) Tractography analysis of 5 white matter bundles and their clinical and cognitive correlates in early-course schizophrenia. Schizophr Bull 42: , 762–771. |
[86] | Wang H , Lenglet C , Akkin T ((2015) ) Structure tensor analysis of serial optical coherence scanner images for mapping fiber orientations and tractography in the brain. J Biomed Opt 20: , 36003. |
[87] | Chad JA , Pasternak O , Salat DH , Chen JJ ((2018) ) Re-examining age-related differences in white matter microstructure with free-water corrected diffusion tensor imaging. Neurobiol Aging 71: , 161–170. |
[88] | Albi A , Pasternak O , Minati L , Marizzoni M , Bartrés-Faz D , Bargalló N , Bosch B , Rossini PM , Marra C , Müller B , Fiedler U , Wiltfang J , Roccatagliata L , Picco A , Nobili FM , Blin O , Sein J , Ranjeva JP , Didic M , Bombois S , Lopes R , Bordet R , Gros-Dagnac H , Payoux P , Zoccatelli G , Alessandrini F , Beltramello A , Ferretti A , Caulo M , Aiello M , Cavaliere C , Soricelli A , Parnetti L , Tarducci R , Floridi P , Tsolaki M , Constantinidis M , Drevelegas A , Frisoni G , Jovicich J ((2017) ) Free water elimination improves test-retest reproducibility of diffusion tensor imaging indices in the brain: A longitudinal multisite study of healthy elderly subjects. Hum Brain Mapp 38: , 12–26 |
[89] | Maier-Hein KH , Westin CF , Shenton ME , Weiner MW , Raj A , Thomann P , Kikinis R , Stieltjes B , Pasternak O ((2015) ) Widespread white matter degeneration preceding the onset of dementia.. Alzheimers Dement 11: , 485–493.e2. |
[90] | Pasternak O , Westin CF , Dahlben B , Bouix S , Kubicki M ((2015) ) The extent of diffusion MRI markers of neuroinflammation and white matter deterioration in chronic schizophrenia. Schizophr Res 161: , 113–118. |
[91] | IBM Corp. Released 2020. IBM SPSS Statistics for Macintosh, Version 27.0. IBM Corp, Armonk, NY. |
[92] | Field A ((2009) ) Discovering statistics using SPSS Sage Publications Ltd, London. |
[93] | Ewers M ((2020) ) Reserve in Alzheimer’s disease. Curr Opin Psychiatry 33: , 178–184. |
[94] | Bastin C , Salmon E ((2014) ) Early neuropsychological detection of Alzheimer’s disease. Eur J Clin Nutr 68: , 1192–1199. |
[95] | Qureshi SU , Long ME , Bradshaw MR , Pyne JM , Magruder KM , Kimbrell T , Hudson TJ , Jawaid A , Schulz PE , Kunik ME ((2011) ) Does PTSD impair cognition beyond the effect of trauma? J Neuropsychiatry Clin Neurosci 23: , 16–28. |
[96] | Rodriguez P , Holowka DW , Marx BP ((2012) ) Assessment of posttraumatic stress disorder-related functional impairment: A review. J Rehabil Res Dev 49: , 649–665. |
[97] | Rona RJ , Jones M , Iversen A , Hull L , Greenberg N , Fear NT , Hotopf M , Wessely S ((2009) ) The impact of posttraumatic stress disorder on impairment in the UK military at the time of the Iraq war. J Psychiatr Res 43: , 649–655. |
[98] | Marshall GA , Sikkes SAM , Amariglio RE , Gatchel JR , Rentz DM , Johnson KA , Langford O , Sun CK , Donohue MC , Raman R , Aisen PS , Sperling RA , Galasko DR ((2020) ) Instrumental activities of daily living, amyloid, and cognition in cognitively normal older adults screening for the A4 Study. Alzheimers Dement (Amst) 12: , e12118. |
[99] | Sperling RA , Donohue MC , Raman R , Sun CK , Yaari R , Holdridge K , Siemers E , Johnson KA , Aisen PS ((2020) ) Association of factors with elevated amyloid burden in clinically normal older individuals. JAMA Neurol 77: , 735–745. |
[100] | Amariglio RE , Sikkes SAM , Marshall GA , Buckley RF , Gatchel JR , Johnson KA , Rentz DM , Donohue MC , Raman R , Sun CK , Yaari R , Holdridge KC , Sims JR , Grill JD , Aisen PS , Sperling RA ((2021) ) Item-level investigation of participant and study partner report on the cognitive function index from the A4 study screening data. J Prev Alzheimers Dis 8: , 257–262. |
[101] | Koychev I , Galna B , Zetterberg H , Lawson J , Zamboni G , Ridha BH , Rowe JB , Thomas A , Howard R , Malhotra P , Ritchie C , Lovestone S , Rochester L ((2018) ) Aβ42/Aβ40 and Aβ42/Aβ38 ratios are associated with measures of gait variability and activities of daily living in mild Alzheimer’s disease: A pilot study. J Alzheimers Dis 65: , 1377–1383. |
[102] | Schuitevoerder S , Rosen JW , Twamley EW , Ayers CR , Sones H , Lohr JB , Goetter EM , Fonzo GA , Holloway KJ , Thorp SR ((2013) ) A meta-analysis of cognitive functioning in older adults with PTSD. J Anxiety Disord 27: , 550–558. |
[103] | Kennis M , Van Rooij SJH , Tromp DPM , Fox AS , Rademaker AR , Kahn RS , Kalin NH , Geuze E ((2015) ) Treatment outcome-related white matter differences in veterans with posttraumatic stress disorder. Neuropsychopharmacology 40: , 2434–2442. |
[104] | Hedges DW , Thatcher GW , Bennett PJ , Sood S , Paulson D , Creem-Regehr S , Brown BL , Allen S , Johnson J , Froelich B , Bigler ED ((2007) ) Brain integrity and cerebral atrophy in vietnam combat veterans with and without posttraumatic stress disorder. Neurocase 13: , 402–410. |
[105] | Daniels JK , Lamke JP , Gaebler M , Walter H , Scheel M ((2013) ) White matter integrity and its relationship to PTSD and childhood trauma - a systematic review and meta-analysis. Depress Anxiety 30: , 207–216. |
[106] | Abe O , Yamasue H , Kasai K , Yamada H , Aoki S , Iwanami A , Ohtani T , Masutani Y , Kato N , Ohtomo K ((2006) ) Voxel-based diffusion tensor analysis reveals aberrant anterior cingulum integrity in posttraumatic stress disorder due to terrorism. Psychiatry Res Neuroimaging 146: , 231–242. |
[107] | Harnett NG , Goodman AM , Knight DC ((2020) ) PTSD-related neuroimaging abnormalities in brain function, structure, and biochemistry. Exp Neurol 330,: , 113331. |
[108] | Ju Y , Ou W , Su J , Averill CL , Liu J , Wang M , Wang Z , Zhang Y , Liu B , Li L , Abdallah CG ((2020) ) White matter microstructural alterations in posttraumatic stress disorder: An ROI and whole-brain based meta-analysis. J Affect Disord 266: , 655–670. |
[109] | O’Doherty DCM , Ryder W , Paquola C , Tickell A , Chan C , Hermens DF , Bennett MR , Lagopoulos J ((2018) ) White matter integrity alterations in post-traumatic stress disorder. Hum Brain Mapp 39: , 1327–1338. |
[110] | Siehl S , King JA , Burgess N , Flor H , Nees F ((2018) ) Structural white matter changes in adults and children with posttraumatic stress disorder: A systematic review and meta-analysis. Neuroimage Clin 19: , 581–598. |
[111] | Olson EA , Cui J , Fukunaga R , Nickerson LD , Rauch SL , Rosso IM ((2017) ) Disruption of white matter structural integrity and connectivity in posttraumatic stress disorder: A TBSS and tractography study. Depress Anxiety 34: , 437–445. |
[112] | Averill CL , Averill LA , Wrocklage KM , Scott JC , Akiki TJ , Schweinsburg B , Southwick SM , Krystal JH , Abdallah CG ((2018) ) Altered white matter diffusivity of the cingulum angular bundle in posttraumatic stress disorder. Mol Neuropsychiatry 4: , 75–82. |
[113] | Koch SBJ , van Zuiden M , Nawijn L , Frijling JL , Veltman DJ , Olff M ((2017) ) Decreased uncinate fasciculus tract integrity in male. J Psychiatry Neurosci 42: , 331–342. |
[114] | McCunn P , Richardson JD , Jetly R , Dunkley B ((2021) ) Diffusion tensor imaging reveals white matter differences in military personnel exposed to trauma with and without post-traumatic stress disorder. Psychiatry Res 298: , 113797. |
[115] | Wiingaard Uldall S , Lundell H , Baaré WFC , Roman Siebner H , Rostrup E , Carlsson J ((2022) ) White matter diffusivity and its correlations to state measures of psychopathology in male refugees with posttraumatic stress disorder. Neuroimage Clin 33: , 102929. |
[116] | Graziano R , Bruce S , Paul R ((2021) ) The corpus callosum and PTSD severity. J Interpers Violence 36: , 7480–7494. |
[117] | Dailey NS , Smith R , Bajaj S , Alkozei A , Gottschlich MK , Raikes AC , Satterfield BC , Killgore WDS ((2018) ) Elevated aggression and reduced white matter integrity in mild traumatic brain injury: A DTI study. Front Behav Neurosci 12: , 118. |
[118] | Nakayama N , Okumura A , Shinoda J , Yasokawa YT , Miwa K , Yoshimura SI ((2006) ) Evidence for white matter disruption in traumatic brain injury without macroscopic lesions. J Neurol Neurosurg Psychiatry 77: , 850–855. |
[119] | Morey RA , Haswell CC , Selgrade ES , Massoglia D , Liu C , Weiner J , Marx CE , Cernak I , McCarthy G ((2013) ) Effects of chronic mild traumatic brain injury on white matter integrity in Iraq and Afghanistan war veterans. Hum Brain Mapp 34: , 2986–2999. |
[120] | Jorge RE , Acion L , White T , Tordesillas-Gutierrez D , Pierson R , Crespo-Facorro B , Magnotta VA ((2012) ) White matter abnormalities in veterans with mild traumatic brain injury. Am J Psychiatry 169: , 1284–1291. |
[121] | Kawai N , Kawanishi M , Kudomi N , Maeda Y , Yamamoto Y , Nishiyama Y , Tamiya T ((2013) ) Detection of brain amyloid β deposition in patients with neuropsychological impairment after traumatic brain injury: PET evaluation using Pittsburgh Compound-B. Brain Inj 27: , 1026–1031. |
[122] | Ponto LLB , Brashers-Krug TM , Pierson RK , Menda Y , Acion L , Watkins GL , Sunderland JJ , Koeppel JA , Jorge RE ((2016) ) Preliminary investigation of cerebral blood flow and amyloid burden in veterans with and without combat-related traumatic brain injury. J Neuropsychiatry Clin Neurosci 28: , 89–96. |
[123] | Hicks AJ , Ponsford JL , Spitz G , Dore V , Krishnadas N , Roberts C , Rowe CC ((2022) ) β-amyloid and tau imaging in chronic traumatic brain injury: A cross-sectional study. Neurology 99: , e1131–e1141. |
[124] | Sydnor VJ , Bouix S , Pasternak O , Hartl E , Levin-Gleba L , Reid B , Tripodis Y , Guenette JP , Kaufmann D , Makris N , Fortier C , Salat DH , Rathi Y , Milberg WP , McGlinchey RE , Shenton ME , Koerte IK ((2020) ) Mild traumatic brain injury impacts associations between limbic system microstructure and post-traumatic stress disorder symptomatology. Neuroimage Clin 26: , 102190. |
[125] | Brown EM , Salat DH , Milberg WP , Fortier CB , McGlinchey RE ((2022) ) Accelerated longitudinal cortical atrophy in OEF/OIF/OND veterans with severe PTSD and the impact of comorbid TBI. Hum Brain Mapp 43: , 3694–3705. |
[126] | O’Doherty DCM , Chitty KM , Saddiqui S , Bennett MR , Lagopoulos J ((2015) ) A systematic review and meta-analysis of magnetic resonance imaging measurement of structural volumes in posttraumatic stress disorder. Psychiatry Res Neuroimaging 232: , 1–33. |
[127] | Logue MW , van Rooij SJH , Dennis EL , Davis SL , Hayes JP , Stevens JS , Densmore M , Haswell CC , Ipser J , Koch SBJ , Korgaonkar M , Lebois LAM , Peverill M , Baker JT , Boedhoe PSW , Frijling JL , Gruber SA , Harpaz-Rotem I , Jahanshad N , Koopowitz S , Levy I , Nawijn L , O’Connor L , Olff M , Salat DH , Sheridan MA , Spielberg JM , van Zuiden M , Winternitz SR , Wolff JD , Wolf EJ , Wang X , Wrocklage K , Abdallah CG , Bryant RA , Geuze E , Jovanovic T , Kaufman ML , King AP , Krystal JH , Lagopoulos J , Bennett M , Lanius R , Liberzon I , McGlinchey RE , McLaughlin KA , Milberg WP , Miller MW , Ressler KJ , Veltman DJ , Stein DJ , Thomaes K , Thompson PM , Morey RA ((2018) ) smaller hippocampal volume in posttraumatic stress disorder: A multisite ENIGMA-PGC study: Subcortical volumetry results from posttraumatic stress disorder consortia. Biol Psychiatry 83: , 244–253. |
[128] | Heppner FL , Ransohoff RM , Becher B ((2015) ) Immune attack: The role of inflammation in Alzheimer disease. Nat Rev Neurosci 16: , 358–372. |
[129] | Jackson J , Jambrina E , Li J , Marston H , Menzies F , Phillips K , Gilmour G ((2019) ) Targeting the synapse in Alzheimer’s disease. Front Neurosci 13: , 735. |
[130] | Wijeratne PA , Alexander DC ((2021) ) Learning transition times in event sequences: The temporal event-based model of disease progression. In. Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics). |
[131] | Mayo CD , Mazerolle EL , Ritchie L , Fisk JD , Gawryluk JR ((2017) ) Longitudinal changes in microstructural white matter metrics in Alzheimer’s disease. Neuroimage Clin 13: , 330–338. |
[132] | Nowrangi MA , Lyketsos CG , Leoutsakos JS , Oishi K , Albert M , Mori S , Mielke MM ((2013) ) Longitudinal, region-specific course of diffusion tensor imaging measures in mild cognitive impairment and Alzheimer’s disease. Alzheimers Dement 9: , 519–528. |
[133] | Kitamura S , Kiuchi K , Taoka T , Hashimoto K , Ueda S , Yasuno F , Morikawa M , Kichikawa K , Kishimoto T ((2013) ) Longitudinal white matter changes in Alzheimer’s disease: A tractography-based analysis study. Brain Res 1515: , 12–18. |
[134] | Yasmin H , Nakata Y , Aoki S , Abe O , Sato N , Nemoto K , Arima K , Furuta N , Uno M , Hirai S , Masutani Y , Ohtomo K ((2008) ) Diffusion abnormalities of the uncinate fasciculus in Alzheimer’s disease: Diffusion tensor tract-specific analysis using a new method to measure the core of the tract. Neuroradiology 50: , 293–299. |
[135] | Park CH , Kim SH , Jung HY ((2019) ) Characteristics of the uncinate fasciculus and cingulum in patients with mild cognitive impairment: Diffusion tensor tractography study. Brain Sci 9: , 377. |
[136] | Morikawa M , Kiuchi K , Taoka T , Nagauchi K , Kichikawa K , Kishimoto T ((2010) ) Uncinate fasciculus-correlated cognition in Alzheimer’s disease: A diffusion tensor imaging study by tractography. Psychogeriatrics 10: , 15–20. |
[137] | Fujie S , Namiki C , Nishi H , Yamada M , Miyata J , Sakata D , Sawamoto N , Fukuyama H , Hayashi T , Murai T ((2008) ) The role of the uncinate fasciculus in memory and emotional recognition in amnestic mild cognitive impairment. Dement Geriatr Cogn Disord 26: , 432–439. |
[138] | Agosta F , Henry RG , Migliaccio R , Neuhaus J , Miller BL , Dronkers NF , Brambati SM , Filippi M , Ogar JM , Wilson SM , Gorno-Tempini ML ((2010) ) Language networks in semantic dementia. Brain 133: , 286–299. |
[139] | Mahoney CJ , Ridgway GR , Malone IB , Downey LE , Beck J , Kinnunen KM , Schmitz N , Golden HL , Rohrer JD , Schott JM , Rossor MN , Ourselin S , Mead S , Fox NC , Warren JD ((2014) ) Profiles of white matter tract pathology in frontotemporal dementia. Hum Brain Mapp 35: , 4163–4179. |
[140] | Serra L , Cercignani M , Basile B , Spano B , Perri R , Fadda L , Marra C , Giubilei F , Caltagirone C , Bozzali M ((2012) ) White matter damage along the uncinate fasciculus contributes to cognitive decline in AD and DLB. Curr Alzheimer Res 9: , 326–333. |
[141] | de Resende E de PF , Xia F , Sidney S , Launer LJ , Schreiner PJ , Erus G , Bryan N , Yaffe K ((2022) ) Higher literacy is associated with better white matter integrity and cognition in middle age. Alzheimers Dement (Amst) 14: , e12363. |
[142] | DeJong NR , Jansen JFA , van Boxtel MPJ , Schram MT , Stehouwer CDA , Dagnelie PC , van der Kallen CJH , Kroon AA , Wesselius A , Koster A , Backes WH , Köhler S ((2023) ) Cognitive resilience depends on white matter connectivity: The Maastricht Study. Alzheimers Dement 19: , 1164–1174. |
[143] | Brichko R , Soldan A , Zhu Y , Wang M-C , Faria A , Albert M , Pettigrew C , BIOCARD Research Team ((2022) ) Age-dependent association between cognitive reserve proxy and longitudinal white matter microstructure in older adults. Front Psychol 13: , 859826. |
[144] | Stone DB , Ryman SG , Hartman AP , Wertz CJ , Vakhtin AA; Alzheimer’s Disease Neuroimaging Initiative ((2021) ) Specific white matter tracts and diffusion properties predict conversion from mild cognitive impairment to Alzheimer’s disease. Front Aging Neurosci 13: , 711579. |
[145] | Kalisch R , Baker DG , Basten U , Boks MP , Bonanno GA , Brummelman E , Chmitorz A , Fernàndez G , Fiebach CJ , Galatzer-Levy I , Geuze E , Groppa S , Helmreich I , Hendler T , Hermans EJ , Jovanovic T , Kubiak T , Lieb K , Lutz B , Müller MB , Murray RJ , Nievergelt CM , Reif A , Roelofs K , Rutten BPF , Sander D , Schick A , Tüscher O , Diest I van , Harmelen AL van , Veer IM , Vermetten E , Vinkers CH , Wager TD , Walter H , Wessa M , Wibral M , Kleim B ((2017) ) The resilience framework as a strategy to combat stress-related disorders. Nat Hum Behav 1: , 784–790. |
[146] | Catani M , Howard RJ , Pajevic S , Jones DK ((2002) ) Virtual in Vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage 17: , 77–94. |
[147] | Schmahmann JD , Pandya DN ((2008) ) Disconnection syndromes of basal ganglia, thalamus, and cerebrocerebellar systems. Cortex 44: , 1037–1066. |
[148] | Fox CJ , Iaria G , Barton JJS ((2008) ) Disconnection in prosopagnosia and face processing. Cortex 44: , 996–1009. |
[149] | Ffytche DH ((2008) ) The hodology of hallucinations. Cortex 44: , 1067–1083. |
[150] | Epelbaum S , Pinel P , Gaillard R , Delmaire C , Perrin M , Dupont S , Dehaene S , Cohen L ((2008) ) Pure Alexia as a disconnection syndrome: New diffusion imaging evidence for an old concept. Cortex 44: , 962–974. |
[151] | Ross ED ((2008) ) Sensory-specific amnesia and hypoemotionality in humans and monkeys: Gateway for developing a hodology of memory. Cortex 44: , 1010–1022. |



