GIST in Third Part of Duodenum Causing Partial Obstruction – Laparoscopic Management
A B S T R A C T
Background: Gastrointestinal Stromal Tumor (GIST) arises from the interstitial cells of Cajal. Stomach is the most common site of GIST. The usual presentations of GIST are gastrointestinal bleeding and abdominal pain. GISTs in the duodenum are uncommon. Duodenal obstruction due to GIST is even more uncommon. Duodenal GISTs are usually detected on endoscopy, however they can be missed on routine endoscopy if they are located in the third part of duodenum. Computed Tomography (CT) of abdomen is useful in determining the exact size, location and extent of tumor. In the absence of metastases, surgical excision is the mainstay of treatment.
Case Presentation: We present a case of a duodenal GIST in the 3rd part of duodenum, causing partial obstruction to the duodenal lumen and causing recurrent abdominal pain and vomiting. Although technically challenging, we successfully excised this lesion laparoscopically. Furthermore, as it was a pedunculated tumor arising from the posterior wall of the duodenum, we performed a novel technique of laparoscopic duodenotomy and stapling of pedunculated tumor at the base and primary closure, rather than the conventional technique of wedge or segmental resection.
Conclusion: Third part of the duodenum is a difficult location to access surgically and excision of tumors in this location is usually performed by the open method because of the technical difficulties of laparoscopic surgery in this location. However, with adequate expertise and advanced laparoscopic skills, laparoscopic excision of tumors in the third part of duodenum can be accomplished with good post-operative outcomes.
Keywords
Duodenum, gastrointestinal stromal tumor, laparoscopy, excision
Introduction
Gastrointestinal Stromal Tumors (GIST) are mesenchymal tumors arising from the interstitial cells of Cajal located in the muscular layer of the gastrointestinal tract. The most common site of GIST is stomach. GISTs in duodenum are rare. In the absence of metastatic disease, surgery is the preferred treatment. In the stomach laparoscopic resections are relatively simple, but in duodenum they can be challenging, however with increasing expertise in the field of laparoscopy, resection with negative margins can be achieved even in lesions at difficult locations within the duodenum.
Case Report
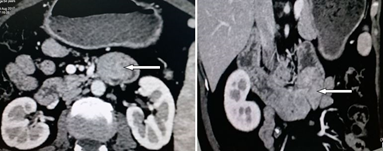
A 55-year-old lady presented with recurrent vomiting and colicky abdominal pain for 1 year. Pain was worse post-prandial and was relieved after vomiting. There was no history of jaundice or fever. Her abdominal examination was unremarkable. Upper gastrointestinal endoscopy up to the second part of the duodenum was normal. Computed Tomography (CT) of abdomen showed a 4 cm × 3 cm polypoidal lesion, possibly a GIST in the third part of duodenum occupying almost the entire lumen with grossly dilated stomach and proximal duodenum suggestive of obstruction (Figure 1).
Figure 1: CT abdomen (Axial section – left, Coronal section – right) showing tumor (denoted by arrow) in the third part of duodenum occupying entire lumen with grossly dilated stomach.
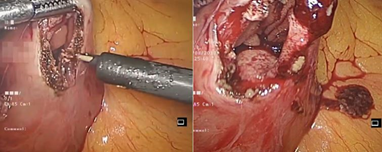
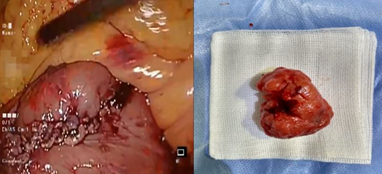
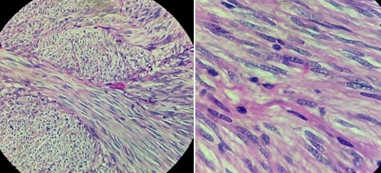
In view of the above, we proceeded with laparoscopy. At laparoscopy, after cephalad retraction of greater omentum and transverse colon, the tumor was felt in the 3rd part of duodenum. Ligament of Treitz was taken down and the 3rd and 4th part of the duodenum were fully mobilized. A longitudinal duodenotomy in the 3rd part of the duodenum was performed. A large pedunculated tumor was seen arising from the posterior wall (Figure 2). As the tumor was pedunculated, it was stapled at the base using endoscopic linear stapler (blue cartridge) (Figure 3). Duodenotomy was closed transversely to avoid luminal narrowing using 2’0 polydioxanone sutures and the specimen was retrieved in a bag (Figure 4). Histopathology showed the lesion to be a Grade 1 GIST, spindle cell subtype, mitotic index of 1 per 10 high power field, with uninvolved margins (Figure 5).
Figure 2: Laparoscopic view of longitudinal duodenotomy in the third part of duodenum and visibility of tumor arising from the posterior wall.
Figure 3: Pedunculated tumor arising from posterior wall lifted well to expose normal duodenal mucosa and then stapled at its base using endoscopic linear stapler.
Figure 4: Transverse closure of duodenotomy (left) and excised specimen (right).
Figure 5: Histopathology showing spindle cells with eosinophilic cytoplasm (40 x microscopic view - left) with no significant atypia or increased mitosis (100 x microscopic view - right).
Patient’s post-operative period was uneventful. She was kept nil by mouth with nasogastric tube drainage for the first 24 hours. As her nasogastric tube output was insignificant, it was removed on the second day, and she was introduced fluids orally and gradually progressed to solid diet over the next few days. She was discharged on the 5th post-op day in stable condition.
Discussion
Gastrointestinal stromal tumors (GISTs) are mesenchymal tumors that arise from the interstitial cells of Cajal or their precursors located throughout the muscular wall of the gastrointestinal tract. They are most commonly found in the stomach (50-60%) followed by small bowel (25%), colon and rectum (10%), while only 3-5% of GISTs occur in the duodenum. Duodenal GISTs most frequently involve the second part of the duodenum, followed in order by the third, fourth and first [1, 2]. The most common clinical presentations are bleeding and abdominal pain [3, 4]. Rarely, they present with back pain or jaundice. Upper gastrointestinal endoscopy is the most common diagnostic tool for duodenal GISTs, however, like in our patient, unless the endoscopist has prior knowledge through imaging, the scope is usually not advanced beyond the ampulla and tumors in the third part of duodenum often get missed.
CT is helpful in determining the size, extent and extra-luminal component of the GIST. On CT, GISTs are hypervascular with cystic and necrotic components. Smaller tumors appear as sharply marginated smooth masses with moderate to high contrast enhancement. GISTs in the duodenum do not differ from other GISTs in immunohistochemical reaction. Most of them express CD -117 (c-kit), CD-34 and PDGFRA. Most of the duodenal GISTs are small in size with spindle cell differentiation, lower mitotic count with a median count of 5/50 HPF. For these reasons, duodenal GISTs usually have a better prognosis compared to gastric and small bowel GISTs. As infiltrative characteristics and lymph nodal metastatic potential are rare with GISTs, the treatment of choice for non-metastatic GISTs is surgery. Disease-free survival rate at 1-3 years after complete surgical resection has been reported to vary from 86% to 100% [4-6].
The surgical approach and technique are dictated by the location of the tumor and the ability to achieve an R0 margin. In the duodenum, wedge resection with primary closure can be performed, however if there is a risk of duodenal luminal narrowing, a segmental resection and duodeno-jejunal anastomosis or a gastro-jejunal anastomosis (for GIST located in the first part of duodenum) is performed. GIST arising in the periampullary region may require pancreaticoduodenectomy [7, 8]. GIST in the 3rd and 4th part of duodenum can be managed by either wedge or segmental resection, however surgery in this area is technically challenging in view of the vital adjacent structures such as Inferior mesenteric vein close to ligament of Treitz, body of pancreas laterally and uncinate process medially, root of the small bowel mesentery with superior mesenteric pedicle. Most of the patients with duodenal GISTs who have undergone the above-described procedures have required a laparotomy. With increasing expertise in minimal access surgery, there are emerging reports of these procedures being performed laparoscopically. Minimally invasive resection performed with clear margins allows low morbidity and quick recovery and has comparable oncological results [9, 10].
Most reports of laparoscopic management of duodenal GISTs in the third part of duodenum are either en-bloc resections such as pancreatico-duodenectomy or wedge resections or segmental resections [9]. To our knowledge, there are no reports of laparoscopic stapled excision for a pedunculated GIST in the third part of the duodenum. We would recommend this novel technique for pedunculated duodenal tumors because it avoids the need for a resection and anastomosis, however we recommend that the surgeon lifts the tumor well (as shown in Figure 3) and staples at the normal duodenal mucosa below the tumor to prevent margin positivity. With this technique, our patient made an uneventful recovery and the histopathology reported completeness of excision of the GIST.
Conclusion
Duodenal GISTs can grow large enough to cause obstruction. Laparoscopic management is feasible in experienced hands even in difficult locations such as the third part of the duodenum. When the lesion is pedunculated, a duodenotomy with stapled excision of the polyp is as effective as wedge or segmental resection.
Author Contributions
JKAJ performed the surgery, reviewed and finalised the manuscript. NJ and HM wrote the initial manuscript. PKR reviewed the manuscript and offered guidance. All authors contributed to the discussion. All authors have approved the final manuscript.
Article Info
Article Type
Case ReportPublication history
Received: Mon 17, Jan 2022Accepted: Fri 28, Jan 2022
Published: Fri 18, Feb 2022
Copyright
© 2023 JKA Jameel. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.SCR.2022.01.08
Author Info
JKA Jameel Nikunj Jain Harshavardan Majety P K Reddy
Corresponding Author
JKA JameelDepartment of Surgical Gastroenterology, Apollo Hospitals, Chennai, Tamil Nadu, India
Figures & Tables

References
1. Yang F, Jin C, Du
Z, Subedi S, Jiang Y et al. (2013) Duodenal gastrointestinal stromal tumor:
Clinicopathological characteristics, surgical outcomes, long term survival and
predictors for adverse outcomes. Am J Surg 206: 360-367. [Crossref]
2. Agaimy A, Wunsch PH
(2006) Gastrointestinal stromal tumours: a regular origin in the muscularis
propria, but an extremely diverse gross presentation. A review of 200 cases to
critically re-evaluate the concept of so-called extra-gastrointestinal stromal
tumours. Langenbecks Arch Surg 391: 322-329. [Crossref]
3. Cavallaro G,
Polistena A, D'Ermo G, Pedullà G, De Toma G (2012) Duodenal gastrointestinal
stromal tumors: review on clinical and surgical aspects. Int J Surg 10:
463-465. [Crossref]
4. Corless CL,
Fletcher JA, Heinrich MC (2004) Biology of gastrointestinal stromal tumors. J
Clin Oncol 22: 3813-3825. [Crossref]
5. Liu Z, Zheng G, Liu
J, Liu S, Xu G et al. (2018) Clinicopathological features, surgical strategy
and prognosis of duodenal gastrointestinal stromal tumors: a series of 300
patients. BMC Cancer 18: 563. [Crossref]
6. Kitamura Y (2008)
Gastrointestinal stromal tumors: Past, present, and future. J Gastroenterol
43: 499-508. [Crossref]
7. Liang X, Yu H, Zhu
LH, Wang XF, Cai XJ (2013) Gastrointestinal stromal tumors of the duodenum:
surgical management and survival results. World J Gastroenterol 19:
6000-6010. [Crossref]
8. Beham A, Schaefer
IM, Cameron S, von Hammerstein K, Füzesi L et al. (2013) Duodenal GIST: a
single center experience. Int J Colorectal Dis 28: 581-590. [Crossref]
9. Kim DJ, Lee JH, Kim W (2014) Laparoscopic resection for 125 gastroduodenal submucosal tumors. Ann Surg Treat Res 86: 199-205. [Crossref]
10. Zioni T, Dizengof V, Kirshtein B (2017) Laparoscopic resection of duodenal gastrointestinal stromal tumour. J Minim Access Surg 13: 157-160. [Crossref]
