Giant De-Differentiated Liposarcoma of the Ascending Colon – A Rare Entity
A B S T R A C T
Background: Liposarcoma is one of the common malignant mesenchymal tumors of the retroperitoneum and soft tissue of the extremities. Liposarcoma primarily arising from the colon is extremely rare. De-differentiated liposarcoma, a high grade variant has high local recurrence and distant metastatic potential. At present no standard treatment guidelines are established for the colonic liposarcoma in view of the rarity of the condition.
Case Presentation: In this article we report a case of 46-year-old female who presented with a large right sided abdominal lump. On cross sectional imaging, she was found to have a large heterogenous exophytic mass arising from the ascending colon. Colonoscopy showed a submucosal lesion of the ascending colon with luminal narrowing. She underwent a right radical hemicolectomy. Histopathology of the resected specimen was reported as a de-differentiated liposarcoma arising from the ascending colon, which is an extremely rare occurrence. Patient made an uneventful recovery and is under follow-up.
Conclusion: Liposarcoma is a rare disease of large intestine. It should be considered in the differential diagnosis when there is a large, submucosal colonic swelling with no lymph nodal or peritoneal disease. Complete surgical resection with clear margins is the standard of treatment. Evidence on the benefits of adjuvant chemotherapy and radiotherapy in improving survival in liposarcoma of colon is currently evolving.
Keywords
Liposarcoma, ascending colon, de-differentiated, retroperitoneal, abdominal mass
Introduction
Liposarcoma is a malignant tumor of mesenchymal origin. It accounts for approximately 20% of all the soft tissue sarcomas and affects both sexes equally. It commonly occurs in the retroperitoneum and soft tissues of the extremities; however, it has also been reported to arise from the gastrointestinal tract. Within the gastrointestinal tract, colonic liposarcomas are extremely rare, with fewer than 50 cases reported in world literature, ever since the first case of primary colonic liposarcoma was reported by Wood and Morgenstern in 1989 [1]. Herein, we report a case of a giant liposarcoma of the ascending colon presenting as a large palpable abdominal lump clinically, causing significant abdominal symptoms, which was successfully treated by a radical resection.
Case Report
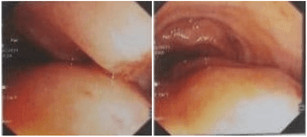
A 46-year-old female, a known case of systemic hypertension and with a past surgical history of laparoscopic cholecystectomy 15 years earlier for symptomatic cholelithiasis, presented with a lump in the right side of the abdomen for 1 month, associated with continuous dull aching type of pain. There was no history of fever, jaundice, vomiting, change in bowel habits, malena or haematochezia. On clinical examination a large, firm, non-tender mass was palpable in the right hypochondrium extending to the right lumbar and iliac regions. Routine blood investigation parameters were within normal limits. Ultrasonography of abdomen suggested a large heteroechoic mass in the right lumbar region arising from the ascending colon. Colonoscopy showed no mucosal lesions but a large submucosal swelling at the hepatic flexure with luminal narrowing (Figure 1).
Figure 1: Colonoscopy images suggestive of submucosal lesion in ascending colon occupying majority of the colonic lumen.
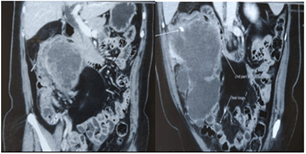
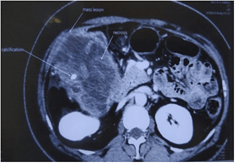
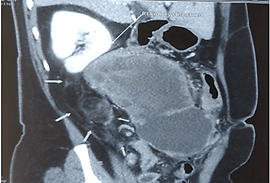
Contrast enhanced CT abdomen revealed a lobulated exophytic mass lesion arising from the inferior wall of hepatic flexure of colon measuring 16.3 x 10.4 x 10.6 cm with moderate peripheral enhancement and central areas of necrosis with specks of calcification in the periphery, suspicious of gastrointestinal stromal tumor (GIST) (Figure 2). The mass was displacing the right kidney cranially, duodenum medially and abutting the anterior abdominal wall with well-preserved planes (Figure 3). Heterogeneously enhancing enlarged lymph nodes were seen around the mass and in ileocaecal mesentery with the largest lymph node measuring 11 x 9 mm. In addition, there was also a fat density lesion in the retroperitoneum at the right peri-nephric space, extending caudally upto the right common iliac artery bifurcation, suggestive of lipoma (Figure 4).
Figure 2: CECT Abdomen showing large exophytic lesion arising from the inferior wall of hepatic flexure of colon, compressing the second part of duodenum.
Figure 3: CECT abdomen showing calcification and necrosis within the tumor.
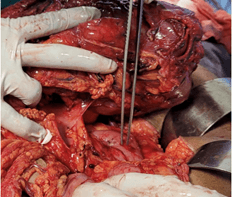
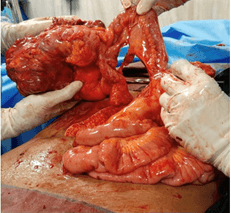
The patient underwent laparotomy, radical right hemicolectomy and excision of the retroperitoneal mass. Upon entering the abdomen, a large multilobulated tumor situated in the ascending colon and its mesocolon with a dimensions of approximately 15 x 14 x 10 cm were noted. There were no ascites, peritoneal disease or liver deposits. Right colon was carefully mobilized taking utmost care to identify and preserve the displaced right ureter. Superiorly the mass was in close proximity to the second part of the duodenum and the pancreatic uncinate process, from which it was safely dissected out (Figure 5). A radical dissection of several enlarged lymph nodes along the ileocolic and middle colic vessels up to the root of the mesocolon was performed (Figure 6). Terminal ileum and distal transverse colon were divided using linear staplers. The giant colonic mass and the retroperitoneal lipoma was excised (Figure 7).
Figure 4: CECT abdomen showing displacement of right kidney by the colonic mass and large retroperitoneal lipomatous tumor.
Figure 5: Giant right colonic mass safely dissected off second part of duodenum (indicated by the forceps).
Figure 6: Terminal ileum after complete mobilisation of the right colon and transection of the transverse colon (just prior to ileal transection).
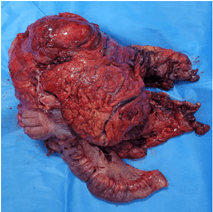
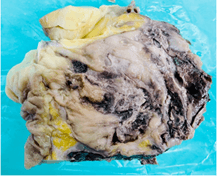
A side-to-side ileo-transverse stapled anastomosis was fashioned. Patient was extubated immediately post-surgery and observed in the high dependency unit for the first 24 hours, after which she was transferred to the ward. Her post-operative course was uncomplicated. She was slowly introduced oral fluids and diet as clinically indicated and was discharged on the 5th post-operative day. Macroscopic examination of the specimen revealed a globular mass of 14.5 x 14 x 9.2 cm with varied grey-white to dark brown fleshy cut surface identified in the pericolic fat invading the subserosa of ascending colon (Figure 8).
Figure 7: Resected specimen.
Figure 8: Gross macroscopic picture showing a relatively well circumscribed tumor in pericolic fat adherent to colonic wall having a grey-brown fleshy cut surface.
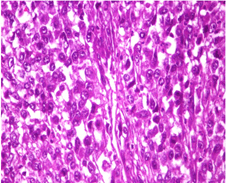
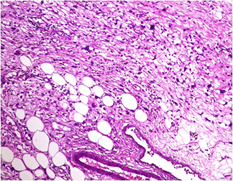
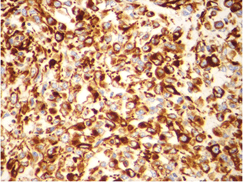
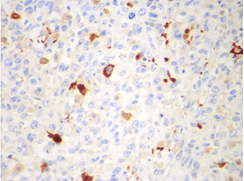
Microscopic examination showed sheets of markedly pleomorphic epithelioid to spindle cells with nucleomegaly, vesicular nuclei and prominent nucleoli. Foci of necrosis with atypical mitosis and mitotic count of 40-45/10 hpf was noted (Figure 9). This tumor was seen as an abrupt transition from a relatively better differentiated neoplasm composed of adipocytes of variable sized with occasional lipoblasts (Figure 10). Background stroma showed fine vascular channels, fibrosis and occasional lymphoid aggregates. Proximal and distal resection margins were clear and 22 pericolic lymph nodes were identified in the specimen, all of them showed reactive changes only. On Immunohistochemical analysis, tumor cells were diffusely positive for vimentin (Figure 11) and a few cells positive for S100 (Figure 12). The tumor cells were negative for C-Kit, DOG-1, CD 34, Desmin and SMA. Above features on histopathological examination and Immunohistchemistry were suggestive of a de-differentiated liposarcoma, and the pathological staging was pT3, N0. On microscopy, the perinephric lipomatous lesions had variable proportions of atypical spindle cells, adipocytes, lipoblasts, pleomorphic multinucleated cells with collagenous and myxoid extracellular matrix and were reported as atypical spindle cell/pleomorphic lipomatous tumor.
Figure 9: Microscopic picture showing poorly differentiated areas with tumor cells exhibiting nucleomegaly, vesicular nuclei and prominent nucleoli (H and E stain, x 400 magnification).
Figure 10: Microscopic picture showing better differentiated areas with varying sized adipocytic cells amidst pleomorphic spindle cells (H and E stain, x 400 magnification).
Figure 11: Microscopic picture showing diffuse positivity of vimentin immunostain in the tumor cells (x 400 magnification).
Figure 12: Microscopic picture showing S100 positivity in few tumor cells (x400 magnification).
Discussion
Liposarcoma is a variety of soft tissue sarcoma that is usually reported from retroperitoneum and soft tissue extremities. Liposarcomas can occur in the gastrointestinal tract too, however they are less frequent. Most of the gastrointestinal liposarcomas involve the gut secondarily. Primary gastrointestinal liposarcomas are rare. They occur commonly between the age of 40 and 60 years with an equal sex distribution. In the large intestine, ascending colon with its mesentery is the most common location for liposarcoma [2]. Clinical presentation of liposarcoma of colon differs widely from being entirely asymptomatic to presenting with a palpable abdominal mass or with nonspecific symptoms like abdominal pain, diarrhoea, weight loss, anemia, haematochezia and constipation.
According to the World Health Organization classification of soft tissue sarcomas, there are 5 types of liposarcomas: well differentiated, dedifferentiated, myxoid, pleomorphic, and mixed type [3]. In the fourth edition of WHO classification in 2013, mixed type has been removed as most of these are unusual type of dedifferentiated liposarcomas [4]. The fifth edition of WHO classification in 2020 added rare and aggressive myxoid pleomorphic liposarcoma which commonly occurs in children and adolescents. It has histologic features from both conventional myxoid liposarcoma and pleomorphic liposarcoma [5]. The well-differentiated type occurs in 40-45%, dedifferentiated type in 15-20%, myxoid in 20-30% and the pleomorphic in 5-10% of all the liposarcomas [6]. The different liposarcoma subtypes have different anatomical distribution. The myxoid and pleomorphic liposarcoma mostly present in the extremities, whereas well differentiated and dedifferentiated liposarcoma occur predominantly in the retroperitoneum.
The most commonly used primary investigation is contrast enhanced computerised tomography (CT) scan of the abdomen and pelvis. The features of liposarcoma on CT scan depend on the amount and distribution of fat in the tumor. On CT scan, liposarcoma has features of inhomogeneous attenuation, poor margination, CT numbers greater than the normal patient fat and irregular contrast enhancement [7]. Well differentiated liposarcoma has predominantly adipose tissue mass with small (< 2 cm) foci of globular non-lipomatous tissue. Calcifications may also be present within the lesion. Larger non-lipomatous foci of more than 2 cm may indicate dedifferentiated liposarcoma. On MR imaging dedifferentiated liposarcoma has low to intermediate signal intensity on T1-weighted imaging and higher signal intensity on T2-weighted imaging [8].
Lipoblasts which are morphologically immature fat cells are characterized by the presence of hyperchromatic indented nucleus and mono to multivacuolated cytoplasm. Lipoblasts were previously considered as diagnostic for liposarcoma. However, lipoblasts can be present in benign lesions like chondrolipoma and spindle cell lipoma too. Hence presence of lipoblasts is not a prerequisite for the diagnosis of liposarcoma [9]. Well differentiated liposarcoma, a low-grade tumor is composed of mature adipocytes with a variable amount of hyperchromatic atypical spindle cells and fibrous bands. Dedifferentiated liposarcoma, a high-grade tumor develops as a focal outgrowth in a preexisting well differentiated lesion. The dedifferentiation in well differentiated lesion commonly occur in the retroperitoneum compared to the extremities [10]. Both well differentiated and dedifferentiated liposarcoma show high level amplifications of chromosome 12q13-15, which includes the CDK4 and MDM2 genes [11]. The amplification of MDM2 gene causes impairment of P53 gene, which leads to tumor proliferation. In addition, dedifferentiated liposarcoma shows gain of 1q23, 6q23, 20q13, or 12q24 mutation. On immunohistochemistry dedifferentiated liposarcoma is usually negative for CD 117 and CD 34 markers and positive for S100 [12].
Complete surgical excision with clear margins is the gold standard for the treatment of liposarcoma. Lymph node metastases from sarcoma is very rare, seen in 1.6-12% of cases. The retroperitoneal dedifferentiated liposarcoma is associated with an 83% local recurrence rate and 30% distant recurrence rate at 3 years [13]. The tumor size (> 15 cm), non-extremity primary site, and disease of high histologic grade are adverse prognostic factors [14].
It has been reported that the 5-year overall survival rate in the preoperative radiotherapy group was higher (62%) compared to no radiotherapy group (54%) in primary retroperitoneal liposarcomas [15]. In a subgroup analysis of liposarcoma patients in EORTC 62092 STRASS trial, 10% recurrence-free survival benefit was identified in radiotherapy plus surgery group compared to surgery alone group [16]. Adjuvant chemotherapy using doxorubicin has marginal benefit in improving disease free survival and overall survival in well differentiated retroperitoneal liposarcomas. Addition of ifosfamide to doxorubicin has also shown to be of added benefit [17, 18]. However, such benefits are not clearly proven for de-differentiated liposarcomas. Furthermore, to our knowledge, no such evidence is available for primary colonic liposarcomas. Our patient was seen by the medical oncologist and was offered doxorubicin-based adjuvant chemotherapy, but she declined due to personal reasons.
Conclusion
Colon is a rare site for soft tissue sarcoma. Liposarcoma should be considered in differential diagnosis when there is an unusually large tumor arising from the colon in the absence of ascites or peritoneal disease on imaging and when colonoscopy reveals a submucous swelling with no mucosal abnormalities. Surgical excision with clear margins is the standard of care. De-differentiated liposarcomas of the retroperitoneum are high risk tumors and have been reported to have a poor prognosis, however the prognosis of these liposarcomas in the colon is not known, due to the rarity of the condition. Based on available data, it appears that adjuvant radiotherapy and doxorubicin-based chemotherapy may have some benefit in colonic liposarcomas.
Author Contributions
NS and DR wrote the initial manuscript. PKR reviewed the manuscript. LM performed the histopathological examination and reviewed the manuscript. JKAJ performed the surgery and reviewed the manuscript. All authors contributed to the discussion. All authors have read and approved the final manuscript.
Article Info
Article Type
Case ReportPublication history
Received: Tue 22, Jun 2021Accepted: Sat 03, Jul 2021
Published: Thu 29, Jul 2021
Copyright
© 2023 JKA Jameel. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.SCR.2021.07.11
Author Info
N Sunil D Raghavendra L Mekala PK Reddy JKA Jameel
Corresponding Author
JKA JameelDepartment of Surgical Gastroenterology, Apollo Hospitals, Chennai, Tamil Nadu, India
Figures & Tables
References
1. Wood DL,
Morgenstern L (1989) Liposarcoma of the ileocecal valve: a case report. Mt
Sinai J Med 56: 62-64. [Crossref]
2. Serafini L, Lauro A, Eusebi LH, Vaccari S, Pirini MG et al. (2020)
Dedifferentiated Liposarcoma of the Descending Colon: A Case Report and Review
of the Literature. Dig Dis Sci 65: 1643-1651. [Crossref]
3. Fletcher CD, Unni
KK, Mertens F (2020) World Health Organization Classification of Tumors. In
Pathology and Genetics of Tumors of Soft Tissueand Bone. IARC Press
2002: 227-232.
4. Fletcher CDM (2014)
The evolving classification of soft tissue tumours - an update based on the new
2013 WHO classification. Histopathology 64: 2-11. [Crossref]
5. Choi JH, Ro JY
(2021) The 2020 WHO Classification of Tumors of Soft Tissue: Selected Changes
and New Entities. Adv Anat Pathol 28: 44-58. [Crossref]
6. Lee ATJ, Thway K,
Huang PH, Jones RL (2018) Clinical and Molecular Spectrum of Liposarcoma. J
Clin Oncol 36: 151-159. [Crossref]
7. Friedman AC,
Hartman DS, Sherman J, Lautin EM, Goldman M (1981) Computed tomography of
abdominal fatty masses. Radiology 139: 415-429. [Crossref]
8. Murphey MD, Arcara
LK, Fanburg Smith J (2005) From the archives of the AFIP: imaging of
musculoskeletal liposarcoma with radiologic-pathologic correlation. Radiographics
25: 1371-1395. [Crossref]
9. Hisaoka M (2014)
Lipoblast: morphologic features and diagnostic value. J UOEH 36:
115-121. [Crossref]
10. McCormick D,
Mentzel T, Beham A, Fletcher CD (1994) Dedifferentiated liposarcoma.
Clinicopathologic analysis of 32 cases suggesting a better prognostic subgroup
among pleomorphic sarcomas. Am J Surg Pathol 18: 1213-1223. [Crossref]
11. Binh MBN, Sastre Garau X, Guillou L, de Pinieux G, Terrier P et al. (2005) MDM2 and
CDK4 immunostainings are useful adjuncts in diagnosing well-differentiated and
dedifferentiated liposarcoma subtypes: a comparative analysis of 559 soft
tissue neoplasms with genetic data. Am J Surg Pathol 29: 1340-1347. [Crossref]
12. Bill KLJ, Garnett
J, Meaux I, Ma X, Creighton CJ et al. (2016) SAR405838: A Novel and Potent
Inhibitor of the MDM2:p53 Axis for the Treatment of Dedifferentiated
Liposarcoma. Clin Cancer Res 22: 1150-1160. [Crossref]
13. Singer S, Antonescu
CR, Riedel E, Brennan MF (2003) Histologic subtype and margin of resection
predict pattern of recurrence and survival for retroperitoneal liposarcoma. Ann
Surg 238: 358-370. [Crossref]
14. Lahat G, Tuvin D, Wei C, Anaya DA, Bekele BN et al. (2008) New
perspectives for staging and prognosis in soft tissue sarcoma. Ann Surg
Oncol 15: 2739-2748. [Crossref]
15. Nussbaum DP,
Rushing CN, Lane WO, Cardona DM, Kirsch DG et al. (2016) Preoperative or
postoperative radiotherapy versus surgery alone for retroperitoneal sarcoma: a
case-control, propensity score-matched analysis of a nationwide clinical
oncology database. Lancet Oncol 17: 966-975. [Crossref]
16. Bonvalot S, Gronchi
A, Le Péchoux C, Swallow CJ, Strauss D et al. (2020) Preoperative radiotherapy
plus surgery versus surgery alone for patients with primary retroperitoneal
sarcoma (EORTC-62092: STRASS): a multicentre, open-label, randomised, phase 3
trial. Lancet Oncol 21: 1366-1377. [Crossref]
17. Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A et al. (2008) A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer 113: 573-581. [Crossref]
18. Koliou P, Karavasilis V, Theochari M, Pollack SM, Jones RL et al. (2018) Advances in the treatment of soft tissue sarcoma: focus on eribulin. Cancer Manag Res 10: 207-216. [Crossref]
