Abstract
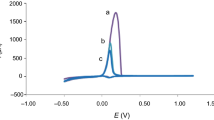
In this work, we report the effect of triazole stabilizers: aminotriazole (ATA), benzotriazole (BTA), and tolytriazole (TTA) on the electroless deposition of copper thin film using xylitol containing copper methanesulphonate bath. Eco-friendly glyoxylic acid was used as reducing agent instead of p-formaldehyde which is carcinogenic. Potassium hydroxide was used for adjusting the pH, since it has the advantage of preventing the formation of insoluble byproducts in electroless bath, which is possible when NaOH is used. The electroless bath was optimized by addition of 1 ppm concentration of stabilizers, at 45°C and pH of 13.25 ± 0.25. Surface roughness, crystallite size and specific surface area of copper deposits were studied by the atomic force microscopy and X-ray diffraction. The anodic peak potential and corrosion current values were analyzed by the cyclic voltammetry and the Tafel plot. ATA was found to inhibit copper deposition while both BTA and TTA accelerated it. The crystallite size and surface roughness values were found to be less than 100 nanometer (nm) compare to the xylitol plain bath. TTA resulted in a better surface, structural, physical and electrochemical property of copper deposition than ATA and BTA.
Similar content being viewed by others
References
Brenner, A.R. and Riddell, G.E., J. Res. Natl. Bur., 1946, vol. 37, pp. 31–34.
Narcus, H., Met. Finish., 1947, vol. 45, pp. 64–67.
Brenner, A., in Symp. on Electroless Nickel Plating, West Conshohocken: Am. Soc. Test. Mater., 1959, no. 265, pp. 1–2.
Saubestre, E.B., J. Electrochem. Soc., 1959, vol. 106, no. 4, pp. 305–309.
Schlesinger, M. and Paunovic, M., Modern Electroplating, New York: Wiley, 2000, vol.14.
Schlesinger, M. and Paunovic, M., Modern Electroplating, New York: Wiley, 2010, 5th ed.
Advances in Electrochemical Science and Engineering, Alkica, R.C. and Kolb, D.M., Eds., New York: Wiley, 2002, vol. 7, pp. 225–270.
Balci, S., Bittner, A.M., Hahn, K., Scheu, C., et al., Electrochim. Acta, 2006, vol. 51, pp. 6251–6257.
Rohan, J.F., Casey, D.P., Ahern, B.M., Rhen, F.M.F., et al., Electrochem. Commun., 2008, vol. 10, pp. 1419–1422.
Alkharafi, F.M., El-Shamy, A.M., and Ateya, B.G., Int. J. Electrochem. Sci., 2009, vol. 4, pp. 1351–1364.
Ababneh, A., Sheban, M., Abu-Dalo, M., and Andreescu, S., Jordan J. Civil Eng., 2009, vol. 3, pp. 91–102.
Antonijevic, M.M. and Petrovic, M.B., Int. J. Electrochem. Sci., 2008, vol. 3, pp. 1–28.
Wu, X., Chou, N., Lupher, D., and Davis, L.C., Proc. 1998 Conf. on Hazardous Waste Research, Snowbird, 1998, vol. 9, pp. 374–382.
Kahled, K.F., Electrochim. Acta, 2009, vol. 54, pp. 4345–4352.
Fox, P.G., Lewis, G., and Boden, P.J., Corros. Sci., 1979, vol. 19, pp. 457–467.
Chadwick, D. and Hashemi, T., Corros. Sci., 1978, vol. 18, pp. 39–51.
Cho, S.-J., Nguyen, T., and Boo, J.-H., J. Nanosci. Nanotechnol., 2011, vol. 11, pp. 5328–5333.
Khaled, K.F., Fadl Allah, S.A., and Hammouti, B., Mater. Chem. Phys., 2009, vol. 117, pp. 148–155.
Yu, L., Guo, L., Preisser, R., and Akolkar, R., J. Electrochem. Soc., 2013, vol. 160, pp. D3004–D3008.
Wu, X. and Sha, W., Synth. React. Inorg., Met.-Org., Nano-Met. Chem., 2008, vol. 38, pp. 292–296.
Karthikeyan, S., Plat. Surf. Finish., 2002, vol. 79, pp. 54–56.
Bhagwat, M., Shah, P., and Ramaswamy, V., Mater. Lett., 2003, vol. 57, pp. 1604–1611.
Ohno, I., J. Mater. Sci. Eng., 1991, vol. 146, pp. 33–49.
Junginger, R. and Elsner, G., J. Electrochem. Soc., 1998, vol. 135, pp. 2304–2308.
Gan, X., Wu, Y., Liu, L., Shen, B., et al., Surf. Coat. Technol., 2007, vol. 201, pp. 7018–7023.
Theivasanthi, T. and Alagar, M., Int. J. Phys. Sci., 2011, vol. 6, pp. 3662–3671.
Balaramesh, P., Venkatesh, P., Rekha, S., and Hemamalini, M., Int. J. Innovative Res. Adv. Stud., 2014, vol. 3, pp. 167–181.
Debye, P. and Scherrer, P., Phys. Z., 1916, vol. 17, pp. 277–283.
Debye, P. and Scherrer, P., Phys. Z., 1917, vol. 18, pp. 291–301.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
About this article
Cite this article
BalaRamesh, P., Venkatesh, P., Thinesh Kumar, R. et al. Influence of triazole stabilizers on the surface morphology of environmentally benign electroless nano copper deposition. Surf. Engin. Appl.Electrochem. 53, 509–514 (2017). https://doi.org/10.3103/S1068375517060023
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1068375517060023