Abstract
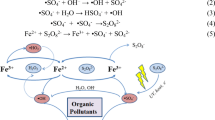
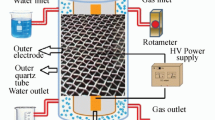
A stainless-steel hollow needle type anode was used in the reactor to treat industrial wastewater by gas-liquid phase of non-thermal plasma by corona discharge. The results showed that the short electrode gap, 1 cm, has a higher plasma energy density which improves the removal of the phenolic derivatives, reaching 100% after about 60 min. The H2O2 concentration was higher in the discharge system when the content of oxygen was increased. The efficiency of the phenol removal by chemical oxygen demand was only 10–31% after 60 minutes. The identified intermediates were catechol, hydroquinone, 1,4-benzoquinone, 2-nitrophenol, 1,2-benzenedicarboxylic acid, diphenylmethanone, 2-methyl-hydroquinone, 2-methyl-1,4-bezoquinone, and trace amounts of organic.
Similar content being viewed by others
References
Sugiarto, A.T. and Sato, M., Thin Solid Films, 2001, vol. 386, pp. 295–299.
Ighigeanu, D.I., Calinescu, I.I., Martin, D.I., and Matei, C.I., AIP Conf. Proc. (Instabul, 2007), Istanbul, 2007.
Lei, L., Zhang, X., and Shen, Y.J., Electrostat., 2008, vol. 66, pp. 16–24.
Farrokhi, M., Mesdahinia, A.R., and Yazdanbakhsh, A.R., and Nasseri, S., Iran. J. Environ. Health, 2004, vol. 1, pp. 12–18.
Chen, Y.S., Zhang, X.C., Dau, Y.C., and Yuan, W.K., Sep. Purif. Technol., 2004, vol. 34, pp. 5–12.
Chang, J., J. Sci. Technol. Adv. Materials., 2004, vol. 2, pp. 571–576.
Hernandez, R., Zappi, M., Colucci, J., and Jones, R., J. Hazard. Materials., 202, vol. 92, pp. 33–50.
Lukes, P., Lupek, M., Babicks, V., and Unka, P., Winerova, G., and Janda, V., Acta Phys. Slovaca., 2003, vol. 53, pp. 423–428.
Hao, X.L., Zhou, M.H., Zhang, Y., and Lei, L.C., Plasma Chem/Plasma Process., 2006, vol. 26, pp. 455–468.
Sun, B., Sato, M., and Clements, J.S., Environ. Sci. and Technol., 2004, vol. 34, pp. 509–513.
Hao, X.L., Zhou, M.H., and Lei, L.C., J. Hazard. Materials, 2007, vol. 141, pp. 475–482.
Pacheco, M,J., Morap, A., Lopes, A., Ciriaco, L., and Goncalves, I., Electrochim. Acta, 2007, vol. 53, pp. 629–636.
Wangs, L. and Jiang, X.J., Hazard. Materials, 2009, vol. 16, pp. 926–932.
Sun, B., Sato, M., and Clements, J.S., J. Electrostat., 1997, vol. 39, pp. 189–202.
Yan, J.H., Du, C.M., Li, X.D., Sun, X.D., Ni, M.J., Cen, K.F., and Cheron, B., Plasma Sources Sci. Technol., 2005, vol. 14, pp. 637–644.
Wang, J., Mei, Y., Liu, V., and Chen, J., J. Environ. Sci., 008, vol. 20, pp. 1304–1311.
Sumka, P., Babixky, V., Clupek, M., Lkes, P., Simek, M., Schmidt, J., and Cernak, M., Plasma Sources Sci. Technol., 1999, vol. 8, pp. 258–265.
Lukes, P., Appleton, A.T., and Locke, B.R., IEEE Trans. Ind. Appl., 2004, vol. 40, pp. 60–67.
Hoeben, W.F.L.M., van Veldhizen, E.M., Rutgers, W.R., Cramers, C.A/M/G., and Krasen, G.M.W., Plasma Sources Sci. Technol., 2000, vol. 9, pp. 361–369.
Shin, W.T., Yiaoumi, S., Tsaoris, C., and Dai, S., Ind. Eng. Chem. Res., 2000, vol. 2000, pp. 4408–4414.
Grymonprs, D.R., Finney, W.C., Clark, R.J., and Locke, B.R., ibid., 2003, vol. 42, pp. 5117–5134.
Lukes, P. and Locke, B.R., Ind. Eng. Chem. Res., 2005, vol. 44, pp. 2921–2930.
Lukes, P. and Locke, B.R., Phys. D: Appl. Phys,, 2005, vol. 38, pp. 4074–4081.
Kubesch, K., Zona, R., Solar, S., and Gehringer, P., 2005, vol. 72, pp. 447–453.
Author information
Authors and Affiliations
Additional information
Original Russian Text © Hsu-Hui Cheng, Shiao-Shing Chen, Kazuharu Yoshizuka, Yung-Chih Chen, 2012, published in Khimiya i Tekhnologiya Vody, 2012, Vol. 34, No. 4, pp. 304–319.
About this article
Cite this article
Cheng, HH., Chen, SS., Yoshizuka, K. et al. Degradation of phenolic compounds in water by non-thermal plasma treatment. J. Water Chem. Technol. 34, 179–189 (2012). https://doi.org/10.3103/S1063455X12040030
Received:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1063455X12040030