Abstract
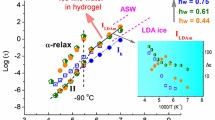
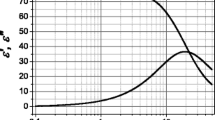
Bound and free water are present in a wide variety of solids, that is single crystals, polymers, and biopolymers, as well as in media with a hydrogen bond network, as in water (2.7 ± 0.1 Å in length). Some objects behave in the same way as two-component systems (open systems) under external influences and demonstrate an abnormal change in properties at the same temperature as water. This paper presents the results of studies of the temperature behavior of the permittivity, conductivity, and conductivity relaxation time of some hydrophilic polymers, crystallohydrates, and ferroelectrics. The analysis of the results showed that temperature anomalies of the selected properties are observed in the vicinity of 20, 35, 65–75, and near 100°C, which are “special” temperatures for water: in the vicinity of 20, 35, 50°C the destruction of clusters of H2O molecules occurs, while at higher temperatures there is a transition of structural water into free water. It is possible that the discrete nature of the diffuse temperature peaks of the properties is due to the presence of discrete energy levels of protons in the matrix–water system, which during stepwise heating (slow kinetics) leads to a rearrangement or destruction of the OH–O hydrogen bond network, as well as the overfilling of the proton levels in the two-minimum potential, the release of deep traps, and changes in the set of current carriers, their mobility, and the trajectories of transport in bulk.






Similar content being viewed by others
REFERENCES
N. D. Gavrilova and V. K. Novik, Moscow Univ. Phys. Bull. 66, 260 (2011). doi 10.3103/S0027134911030076
N. D. Gavrilova and A. A. Davydova, Moscow Univ. Phys. Bull. 68, 143 (2013). doi 10.3103/S0027134913020057
A. V. Vorobyev, N. D. Gavrilova, and A. M. Lotonov, Moscow Univ. Phys. Bull. 69, 175 (2014). doi 10.3103/S0027134914020131
A. V. Vorobyev, N. D. Gavrilova, and A. M. Lotonov, Moscow Univ. Phys. Bull. 70, 175 (2015). doi 10.3103/S0027134915010129
N. D. Gavrilova, O. V. Dimitrova, A. M. Lotonov, N. N. Mochenova, and V. K. Novik, Moscow Univ. Phys. Bull. 63, 127 (2014). doi 10.3103/S0027134908020112
N. D. Gavrilova, I. A. Malyshkina, E. E. Makhaeva, et al., Ferroelectrics 504, 3 (2016). doi 10.1080/00150193.2016.1238284
N. D. Gavrilova, A. V. Vorob’ev, I. A. Malyshkina, E. E. Makhaeva, and V. K. Novik, Polym. Sci. Ser. A 58, 33 (2016). doi 10.1134/S0965545X16010016
N. D. Gavrilova, A. V. Vorobiev, I. A. Malyshkina, and V. K. Novik, Ferroelectrics 478, 88 (2015). doi 10.1080/00150193.2015.1011493
N. D. Gavrilova, V. K. Novik, and I. A. Malyshkina, J. Non-Cryst. Solids 483, 60 (2018). doi 10.1016/j.jnoncrysol.2017.12.056
N. D. Gavrilova, I. A. Malyshkina, V. K. Novik, and A. V. Vorobiev, Ferroelectrics 507, 172 (2017). doi 10.1080/00150193.2017.1283934
Water in Polymers, Ed. by S. P. Rowland (American Chemical Society, 1980).
P. A. Rebinder, Surface Phenomena in Disperse Systems (Nauka, Moscow, 1979).
D. Eisenberg and W Kauzmann, The Structure and Properties of Water (Oxford Univ. Press, 1969).
R. Janoshek, E. G. Weidemann, H. Pfeiffer, and G. Zundel, J. Am. Chem. Soc. 94, 2387 (1972). doi 10.1021/ja00762a032
L. A. Shcherbachenko, N. T. Maksimova, E. S. Komarov, L. I. Ruzhnikov, V. A. Karnakov, E. S. Baryshnikov, D. A. Krasnov, A. A. Troshev, D. S. Baryshnikov, and L. I. Ezhova, Tech. Phys. 57, 1417 (2012). doi 10.1134/S1063784212100209
G. R. Ivanitskii, A. A. Deev, and E. P. Khizhnyak, Phys.-Usp. 57, 37 (2014). doi 10.3367/UFNe.0184.201401b.0043
Z. A. Kats, Production of Dried Vegetables, Potatoes, and Fruits (Legkaya i Pishchevaya Promyshlennost’, Moscow, 1984).
G. A. Lushcheikin, Russ. Chem. Rev. 52, 804 (1983). doi 10.1070/RC1983v052n08ABEH002884
R. Kohlrausch, Pogg. Ann. Phys. Chem. 91, 179 (1854).
G. Williams and D. C. Watts, Trans. Faraday Soc. 66, 80 (1970). doi 10.1039/TF9706600080
A. C. Lopes, C. M. Costa, R. Sabater i Serra, et al., Solid State Ionics 235, 42 (2013). doi 10.1016/j.ssi.2013.01.013
A. V. Milovanov, J. J. Rasmussen, and K. Rypdal, Phys. Lett. A 372, 2148 (2008). doi doi 10.1016/j.physleta.2007.11.025
A. K. Jonscher, Dielectric Relaxation in Solids (Dielectric Press, Chelsea, 1983).
I. D. Nabitovich, N. A. Tsal’, and N. N. Romanyuk, Kristallografiya, No. 4, 985 (1989).
A. K. Kukushkin and S. A. Kuznetsova, Russ. J. Gen. Chem. 77, 2040 (2007).
O. A. Kalmatskaya and V. A. Karavev, Biophysics 60, 843 (2015). doi 10.1134/S0006350915050085
O. A. Kalmatskaya, I. P. Levykina, S. V. Patsaeva, V. A. Karavaev, and V. I. Yuzhakov, Moscow Univ. Phys. Bull. 68, 466 (2013). doi 10.3103/S0027134913060076
L. A. Shcherbachenko, V. S. Borisov, N. T. Maksimova, E. S. Baryshnikov, V. A. Karnakov, S. D. Mar-chuk, Ya. V. Ezhova, and L. I. Ruzhnikov, Tech. Phys. 54, 1372 (2009). doi 10.1134/S1063784209090199
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by K. Gumerov
About this article
Cite this article
Gavrilova, N.D., Malyshkina, I.A. The Influence of Changes in the Structure of Hydrogen Bonds of Water on the Electrophysical Properties of Matrix–Water Systems in Stepwise Heating. Moscow Univ. Phys. 73, 651–658 (2018). https://doi.org/10.3103/S0027134918060127
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0027134918060127