BACKGROUND AND INTRODUCTION
Sport-Related Concussion (SRC) has become a frequent topic of discussion in the sports medicine world. Collegiate football is often the focal point of this discussion due to the high relative risk of SRC. A recent epidemiologic study of SRCs in National Collegiate Athletic Association (NCAA) sports indicated that men’s football has the highest annual rate of SRCs and the highest annual increase in the number of SRCs compared to other collegiate sports,1 with 26.1% of players in a collegiate football program experiencing a concussion at some point in their careers.2 Moreover, nearly 10% of SRCs in collegiate football are recurrent injuries.1 Concussions affect multiple body systems and can physically, cognitively, and psychologically impact a student-athlete. After a concussion, athletes also may face a higher risk of lower extremity injury3 and potential long-term cognitive and neuropsychological consequences.4
To improve concussion management in collegiate athletics, the NCAA implemented a policy in 2010 that addressed the need for a concussion management plan for member institutions. That plan includes removal from play for medical examination of any athlete exhibiting concussion-related symptoms, preclusion of return-to-play (RTP) for any athlete diagnosed with a concussion, and medical clearance to resume play following a concussion diagnosis.5 There was an increase in the number of diagnosed concussions in collegiate football following the implementation of this policy,2 highlighting the need for sensitive testing and quality rehabilitation protocols. The lack of sensitive measures for diagnosis and readiness to RTP can ultimately lead to premature RTP. Baugh et al. studied 730 collegiate football players and found that for every diagnosed concussion, there were 4.125 undiagnosed concussions in an athlete’s career, on average.6 Furthermore, a five-year, retrospective study by Carson et al. found that 43.5% of SRCs returned to play too soon.7
SRCs are challenging to manage due to the underlying pathophysiology. A rapid change in intracranial velocity results in axonal shearing and cerebral edema creating metabolic changes and reduced cerebral blood flow.8 Force directed to the head and neck area can also create cervical spine soft tissue, articular, and ligamentous damage,9 resulting in varying symptoms, recovery times, and clinical trajectories. Presentations can differ greatly amongst athletes, but deficits can include vestibular and ocular impairments, musculoskeletal and cervical impairments, impairments in sensory integration, deficits in motor control, as well as autonomic dysfunction and exertional intolerance.10,11
SRCs are most commonly managed by team athletic trainers (ATCs) and team physicians (MDs).12,13 However, it has been demonstrated that physical therapy can effectively address impairments related to SRC.10 Authors have demonstrated that individuals post-concussion who received individualized physical therapy were 3.91 times more likely to be cleared to RTP after eight weeks,10 and athletes receiving individualized physical therapy within 10-days post-concussion are medically cleared for RTP significantly faster.14 Both cervical and vestibular rehabilitation are recommended by the 5th Consensus Statement of Concussion in Sport13 and pre-morbid risk factors and post-injury prognosticators identifying athletes that are at risk for a prolonged recovery have been established.15,16 Despite this, referral to physical therapy following SRC is frequently at the discretion of team ATCs and MDs as needed for complex vestibular and/or cervical issues or for athletes with persistent symptoms.13
When considering return-to-learn (RTL), improved clarity is also advantageous. The 5th Consensus Statement of Concussion in Sport presents a recommended four-step return-to-school strategy if needed13; however, outside of this recommendation, it provides no definitive criteria for academic accommodations or stepwise progression, the timing of stepwise progression, or multi-disciplinary involvement in addressing cognitive deficits. Although prolonged and complete cognitive rest has been shown to delay recovery,17 Carson et al. found that 44.7% of athletes with SRCs returned to full academic participation too soon, and findings from the Ivy League-Big Ten Epidemiology Study show that athletes who return to full academics too soon take longer to recover from SRC and that improved clarity in the timing of RTL is warranted.7,18 Authors have suggested that impairments in working memory and attentional control can also lead to impairments in lower extremity motor control.19 However, these domains can be improved with targeted interventions, including physical therapy.19
Despite current efforts to better understand and manage SRCs, concussions remain one of the most complex injuries in sports-medicine, making it difficult to identify and implement best practices for a standardized RTP protocol. To develop a protocol that best serves the student-athlete, it is important to understand the landscape of RTP protocols as well as current, evidence-based concussion rehabilitation. Enhancing existing RTP protocols with recently available evidence can reduce premature RTP, improve rehabilitation effectiveness, and expedite recovery for athletes at risk for persistent symptoms.
With the changing landscape of RTP protocols and SRC management, the purpose of this clinical commentary is three-fold: to a) survey the current state of standardization of RTP protocols in collegiate football; b) highlight the development and implementation of a RTP protocol with standardized physical therapy referral and management in an NCAA Division II collegiate football program; and c) describe results of a full-season pilot study, including time to evaluation, time to RTP, rate of re-injury or lower extremity injury, and the clinical significance of protocol implementation. It is hoped that this commentary will aid in the strengthening of RTP protocols by demonstrating the benefit of increased and standardized physical therapy management in RTP. This study has been approved by the Mercer University Institutional Review Board.
CURRENT STANDARDIZATION OF RETURN-TO-PLAY PROTOCOLS
Along with the NCAA’s updated policy regarding SRCs, position statements have been published by several professional organizations with recommendations for SRC management. The most recent Consensus Statement by the Concussion in Sport Group, 2017, presents an updated return-to-sport strategy consisting of a graduated six-step rehabilitation protocol.13 Recommendations for the recognition of a concussion, sideline evaluation and re-evaluation of a concussion, and removal from and reintroduction to play are also discussed.
The National Athletic Training Association (NATA) released a position statement in 2014 with notable recommendations including documentation of an athlete’s concussion evaluation and progression through a RTP protocol, baseline pre-season examinations that include neurocognitive and balance measures for high-risk sports, diagnosis reached by clinical examination and assessment tools, and treatment of athletes on an individual basis.12 The NCAA has also presented a Concussion Safety Protocol Checklist and Concussion Safety Protocol Template to serve as a guide in the development and standardization of RTP protocols.20 The checklist recommends pre-season education presented to athletes, pre-season assessment of concussion history, symptom evaluation, cognitive evaluation, and balance evaluation. A six-step return-to-sport plan is also required.
In 2015, Kerr et al.21 performed a cross-sectional investigation of concussion-related protocols and pre-season assessments that involved all 1,113 NCAA member institutions via surveys sent to all head ATCs. Despite the recommendations for standardized concussion management, results demonstrated that the adherence to recommendations vary by policy and division. Most, but not all, institutions provided concussion education to athletes (95.4%) and had RTP policies (96.6%), while fewer had RTL policies (63.3%).21 Only 83.2% of institutions utilized pre-season neurocognitive testing and only 56.6% incorporated balance assessments.21 The adherence to NATA recommendations also differed by division, with 55.2% of Division I programs complying with baseline measure recommendations compared to 38.2% and 36.1% of Division II and Division III institutions, respectively.21
In 2017, Buckley et al. evaluated the concussion management plans of all 65 Power Five NCAA institutions for compliance with the NCAA’s 2010 concussion policy.22 The overall compliance was 94.3% but varied greatly in detail and length, with one institution only complying with 59.6% of components. The lowest domain for institutional compliance was return-to-learn, with only 86.4% of components addressed. Although the overall compliance rate was high, there continues to be room for improvement.22
Both the consensus statement by the 5th CISG and the NCAA’s Concussion Safety Protocol Checklist recommend referral for multi-discipline management but not until symptoms become “persistent,”13,20 despite evidence that individualized and early onset of physical therapy intervention reduces recovery time and risk for persistent symptoms.10,14 Furthermore, to the authors’ knowledge, there is no literature describing the standardization of concussion rehabilitation or referral to physical therapy within collegiate football.
The variability in RTP policies between schools and divisions can be due to multiple factors, including but not limited to differing education levels, institutional support or financial limitations, or access to medical professionals, including physical therapists specializing in SRC management. However, universities and health care providers need to continue to adapt current policies with current evidence that better protect and serve student-athletes.
RETURN-TO-PLAY PROTOCOL
The implemented RTP protocol, described below, involving multi-disciplinary care from time of injury to full clearance, was developed utilizing recommendations from the 5th CISG consensus statement and NCAA Safety Protocol Checklist, and meets compliance with NCAA legislation. The protocol includes objective baseline neuromotor and neurocognitive testing, standardized sideline assessments, and a six-step RTP progression incorporating individualized physical therapy treatment. Evidence-based risk factors for prognostication were utilized at the time of injury to standardize referral to physical therapy.
It is important to note that the team athletic trainers, team physician, physical therapists, and speech-language pathologist were contracted from a local hospital system and were not university employees. Team ATCs were located within the university’s athletic facility; however, a sports-medicine fellowship-trained physician and physical therapist specialized in concussion management were based in satellite clinics off campus. Educational inservices were provided among disciplines to ensure the proper use of assessments and outcome measures for standardization of care.
Baseline Assessments
All athletes participated in pre-season physical examinations before being cleared to participate. Baseline neurocognitive performance and baseline symptoms were assessed via the Immediate Post-Concussion and Cognitive Testing (ImPACT) battery.23 It has been demonstrated that the ImPACT test battery has a low to moderate test-retest reliability with up to a 26% rate of purposeful underperformance by athletes23; therefore, a multimodal objective assessment is advantageous to assess readiness to RTP. Evidence demonstrates that individuals who are post-concussion have impaired postural stability and sensory integration compared to non-concussed individuals. Therefore, objective baseline assessments of neuromotor function were performed.12,24
A four-condition Clinical Test of Integration in Balance (CTSIB) was performed utilizing a dynamic force plate to gather objective data on baseline postural stability and sensory integration. It has been shown that athletes with a history of concussion can demonstrate clinical deficits in balance long after the initial injury25; therefore, baseline norms were utilized as reference during RTP. Standard deviations for sway values on all four conditions in collegiate athletes were gathered in a study by Moran et al. and were utilized as a second comparison during recovery post-injury.26 The mBESS was not recorded due to concerns over ceiling effects.27 Education regarding symptoms and risks of SRC and the purpose of RTP protocol was also provided during pre-season physicals.
Sideline Assessment of Injury
To increase the sensitivity of clinical diagnosis, ATCs were educated on standardized sideline assessments of vestibular function, including the Vestibular Ocular Motor Screening (VOMS) and modified Concussion Balance Test (mCOBALT), to be performed in conjunction with the Sport Concussion Assessment Tool (SCAT-5). Sideline evaluations also incorporated the assessment of evidence-based negative prognosticators and/or risk factors for a prolonged recovery from SRC. These assessments served as a framework for referral to skilled PT, as athletes identified as at-risk for a prolonged recovery by the ATC and MD were referred to skilled PT.
The VOMS briefly screens vestibulo-ocular and oculomotor function and sensitivity. The VOMS can be used to aid in diagnosis of concussion with any item scored ≥ 2 in sensitivity increasing diagnostic accuracy of a concussion by 46%.10 The addition of the VOMS has been demonstrated to significantly increase the overall sensitivity and diagnostic capability of the SCAT-5.28 To further assess balance if needed, ATCs were educated on the use of the mCOBALT, which is a four-minute exam derived from the CTSIB and is more sensitive in identifying concussion-related neuromotor impairment than the mBESS due to increased vestibular demand.29 On average, only 55% of concussed athletes can complete the exam due to symptom provocation or loss of balance in comparison to 100% of non-concussed athletes.29
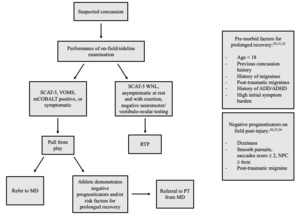
A decision tree was implemented for ATC use during sideline evaluation to identify athletes who are at risk of prolonged recovery from SRC according to evidence-based negative prognosticators post-concussion and/or risk factors (Figure 1). Pre-morbid risk factors include a history of previous concussion, migraines or familial history of migraines, history of anxiety and/or depression, Attention Deficient Disorder (ADD) or Attention Deficit/Hyperactivity Disorder (ADHD), female gender, and age less than18 years.30–32 Athletes with any risk factors have a 35-69% increased risk of recovery greater than 21 days.30
Post-injury negative prognosticators include on-field dizziness, acute vestibulo-ocular abnormalities, post-traumatic migraine, or high initial symptom burden.30,33,34 Those with reports of on-field dizziness have a six-fold increase in risk for a recovery >21 days.34 The Vestibular Ocular Motor Screen can also be utilized to identify the risk of prolonged recovery in collegiate athletes.33 An abnormal score of ≥2 on smooth pursuits, horizontal and vertical saccades, or near-point convergence has been associated with a longer time to RTP.33
Physician Evaluation
To reduce the time from sideline assessment to clinical evaluation by the team physician and physical therapist, a communication portal was created between the ATC, MD, PT, and physical therapy office staff. Once a concussion was suspected, the ATC notified the MD to begin clinical management. It was also communicated by the ATC and MD if an athlete was considered a candidate for skilled PT to coordinate care with the physical therapist. If the MD was able to examine the athlete at the time of injury and deemed PT appropriate due to the risk of a protracted recovery, a referral to PT was placed to aid in the onset of early PT intervention.
The goal was to allow for MD and PT clinical evaluations to be performed within 48 and 96 hours of acute injury. Education on the pathology of concussion, symptoms, recovery trajectory, and RTP protocol was provided during evaluation. Each athlete was educated on limiting cognitive strain, visual strain including phone and computer use, and exertion during the first 48 to 72 hours post-injury. Education on RTL guidelines was also provided during MD evaluation, as described in detail below.
Physical Therapy Evaluation and Management
Physical therapy evaluation assessed constructs in agreement with the American Physical Therapy Association’s clinical practice guideline on concussion management and was directed by each athlete’s presentation.10 Subjective evaluation investigated current symptoms, level of sensitivity, individual functional goals, and the Post-Concussion Symptom Scale. Objective assessments for vestibular and vestibulo-ocular function, cervical spine integrity, exertional tolerance, and neuromotor function were performed, guided by an athlete’s symptom sensitivity.
A Vestibular Ocular Motor Screen was performed to assess for vestibulo-ocular dysfunction. The VOMS incorporates five vestibulo-ocular and oculomotor domains: smooth pursuit, horizontal and vertical saccades, near-point convergence distance, horizontal vestibular ocular reflex (VOR), and visual motion sensitivity (VMS) and has an excellent internal consistency (α = .92)11 This was not compared to sideline performance of the VOMS, which was used to aid in diagnosis, but utilized to identify vestibulo-ocular impairments and sensitivities for guided treatment.
A Motion Sensitivity Quotient (MSQ) was also performed if motion sensitivity was subjectively reported or suspected. The MSQ is designed to identify and measure motion-provoked dizziness via 16 rapid body or head movements and was used clinically to develop an individualized exercise program for motion habituation.35 Both vestibulo-ocular and motion sensitivities were incorporated into later stages of rehabilitation via sport-related movements and drills. If room-spinning vertigo was reported, a screen for benign positional vertigo (BPV), including the Dix-Hallpike Test and Supine Roll Test, was performed for posterior/anterior and horizontal canal BPV, respectively.36
If motion sensitivity or gaze instability was reported, a vestibular head impulse test (HIT) was performed to screen for peripheral vestibular hypofunction. A HIT directly assesses the vestibular-ocular response from the peripheral vestibular system as the clinician rapidly and unpredictably turns the athlete’s head during visual fixation to assess instantaneous corrective eye movements.37 With vestibular hypofunction, the eyes move with the head during an unexpected head turn and must make a corrective movement to re-fix on the target, indicating impaired vestibulo-ocular response gain.
Cervical spine range of motion, accessory mobility, and soft tissue palpation were examined along with assessments for potential cervicogenic headache, cervicogenic dizziness, cervico-ocular dysfunction, and/or motor control impairments. The Cervical Flexion-Rotation test investigates cervicogenic headache and dizziness related to the first and second cervical facet joints by maximally flexing and rotating the upper cervical spine. Provocation of headache or dizziness with upper cervical approximation is considered positive (Sn: 0.91, Sp: 0.90).38 Cervico-ocular dysfunction was assessed via the Smooth-Pursuit Neck Torsion Test, which compares the oculomotor performance and sensitivity of smooth pursuits in neutral and in 45 degrees of rotation. Increased sensitivity and the presence of nystagmus with cervical rotation can indicate upper cervical-related oculomotor dysfunction (Sn: 0.90, So: 0.91).39 Cervical joint position error was investigated if impairments in soft tissue mobility, cervical accessory mobility, or motor control were demonstrated. Cervical joint position error was assessed via the cervical joint relocation test, which utilizes a cervical laser harness to identify the degree of position error and cervical sensorimotor disturbances.39
A post-injury objective CTSIB was performed for comparison to baseline data of balance and sensory integration, with symptom provocation also recorded. An mCOBALT test was not performed at the time of clinical evaluation as this was utilized as a part of the sideline examination to aid in diagnostic sensitivity. An mCOBALT was performed, however, prior to discharge to ensure no neuromotor impairment or vestibular sensitivities arose with more sensitive vestibular demands on balance.
To assess for autonomic dysfunction and/or exertional intolerance, a Buffalo Concussion Exercise Treadmill Test (BCTT) was performed.40 The BCTT gradually increases the intensity of exercise via treadmill incline while vitals and symptoms are assessed every one to two minutes. The test is discontinued if the athlete reaches voluntary exhaustion or if post-concussion symptoms are exacerbated.40 Heart rate was recorded at the onset or exacerbation of symptoms, which is considered the athlete’s heart rate threshold (HRT). If motion sensitivity was identified during vestibular examination, a modified Buffalo Concussion Bike Test (BCBT) was performed on an upright exercise bike to increase test specificity by accounting for potential increases in dizziness from a vertical translation during ambulation.
An individualized home exercise program was prescribed including interventions for cervical spine soft tissue mobility, cervical motor control, and vestibular habituation and/or adaptation. Graded aerobic exercise beginning less than 10 days post-injury has been associated with a faster RTP41; therefore, daily sub-symptom aerobic cardiovascular exercise was prescribed utilizing the established HRT. Both the ATC and MD were informed of an athlete’s impairments, exercise program, and estimated prognosis for recovery.
Athletes continued individualized skilled physical therapy through stage three of the stepwise progression. Frequency was determined by impairments and severity. Interventions could include exercises for cervical spine motor control and proprioception, vestibular adaptation and/or habituation, exertional tolerance, and cervical vestibular ocular integration. Interventions also incorporated dual motor and cognitive task practice throughout all stages. All exercises incorporating vestibular or vestibulo-ocular tasks were performed at an intensity that provoked light to moderate dizziness (4-6 out of 10), or below migraine symptom provocation. Once moderate dizziness was achieved, the athlete was instructed to rest until symptoms returned to baseline to promote vestibular habituation.42 Athletes continued to receive updated exercise programs reflecting their current RTP stage to be performed individually and supervised by ATCs.
Stepwise Return-to-Play
All athletes progressed through a six-step protocol as recommended from the 5th Concussion in Sport Group (CISG) meeting13 (Table 1), supervised by the ATC and PT under direction of the team MD. Stage zero began at the time of injury and included rest for the first 24-48 hours. Athletes then progressed to stage one which included vestibular and cervical exercises provided by a physical therapist and sub-symptom aerobic conditioning guided by HRT identified via the BCTT or BCBT, if exertional intolerance and HRT were found.10 During each physical therapy session, athletes performed supervised aerobic cardiovascular exercise utilizing a treadmill or stationary bike and gradually increased their HR until either a new HRT was established or 70% HR max was achieved. Athletes could then progress to sport-related exercise (Stage 2 of RTP) with achievement of 70% HR max without symptom provocation and no symptoms at rest.
Stage 2 added sport-related body weight exercise, dynamic movement, and running drills supervised by the PT or ATC. Once athletes were able to perform sport-related exercise at moderate intensity (HR 70-80% HR max) without symptom provocation, they progressed to Stage 3, which incorporated non-contact sport-related rills, resistance training, and increased anaerobic demands. Both sport-related exercise and non-contact sport-related drills emphasized position-specific movements and vestibular, vestibulo-ocular, and cervical sensitivities specific to the athlete. A focal point of physical therapy intervention was the incorporation of dual motor and/or cognitive tasks throughout RTP progression.
Dual task is considered the performance of simultaneous motor or cognitive tasks.43 During open sports, such as football, athletes must regulate both internal processes (e.g., joint position sense, vestibular/ocular function) and respond to external cues (e.g., the ball, opponents, teammates) in order to modify movement and perform the intended motor task such as running, cutting, blocking, catching, or tackling.19 The execution of these dual tasks occurs in time-constrained environments necessitating increased processing speed, reaction time, and working memory, all of which may be impaired post-concussion and can remained impaired after clearance to RTP.44,45 Impairments in these cognitive domains post-concussion have been correlated to abnormal LE kinematics when given a dual motor/cognitive task.44–46
During physical therapy sessions, athletes were tasked to perform sport-related exercise or drills with simultaneous performance of vestibular or vestibulo-ocular tasks. These tasks also incorporated attentional control or working memory with time-constraints for processing speed. Examples are demonstrated in supplemental Videos 1 and 2.
Prior to progressing to stage four, athletes must have demonstrated CTSIB sway data within one standard deviation of baseline and no symptoms or errors with performance of the mCOBALT to ensure neuromotor function within normal limits. Athletes must also have completed a readiness assessment performed by a physical therapist including high intensity aerobic and anaerobic exertion, sport-related exercise, and sport-related drills with dual motor and cognitive tasks (Table 1). Athletic training staff then progressed athletes to full-contact practice and full RTP as appropriate. Prior to participating in contact practice or full play, athletes must have demonstrated ImPACT testing results within normal limits, have fully returned to learn, and have received MD clearance.
Return-to-Learn
Although the focus of this commentary is on the introduction of standardized PT in RTP, a brief discussion of RTL is also important due to the lack of congruency amongst RTL protocols. To aid in consistency with RTL in this protocol, all athletes who demonstrated risk for a prolonged recovery were placed on a four-step RTL progression by the team MD. The stepwise progression was based upon the graduated RTL strategy recommended by the CISG13 and included: 1) performance of sub-symptom daily activities, 2) performance of school and other cognitive activities as guided by symptoms and beginning 48-72 hours post injury, 3) return to school part-time as advised by the MD, and 4) return to school full-time as advised by the MD or when the athlete can tolerate a modified schedule without symptom exacerbation, whichever occurred first. RTL progression was then communicated to the ATC, school academic counselor, and PT.
If appropriate, athletes returned to school part-time at 72 hours post-injury, as it has been suggested that strict rest longer than three days can protract recovery.17 Part-time participation could include partial attendance or shortened class periods, use of paper assignments to reduce visual strain, increased time for test taking, or increased breaks for symptom pacing, up to stage 4 of RTL. Athletes could not graduate from the stepwise progression until fully caught up on classwork and exams.
A referral for Speech-Language Pathology (SLP) was recommended if primary symptoms and/or impairments were consistent with the cognitive/fatigue clinical profile as described by Kontos et al.,47 if cognitive strain provoked post-traumatic migraine symptoms, or other cognitive impairments persisted past 10-14 days. Although SLPs are uncommon in traditional concussion management team, speech-language pathology is the second most common referral destination from a specialized concussion clinic, behind physical therapy.48
In the context of concussion management, an SLP examines cognitive processing skills, divided attention, and working memory and provides strategies and skills to aid in active cognitive recovery.48 A communication portal was created including athletic trainers, team physician, and the university academic coordinator in which the SLP would provide insight into cognitive strategies to better individualize the RTL plan. Furthermore, the SLP would communicate to the physical therapist the cognitive domains in which an athlete had deficits, e.g., working memory, divided attention, in order for the PT to incorporate these demands into dual-task interventions.
RESULTS
A pilot study cohort for this approach consisted of 13 athletes who entered the concussion protocol, were identified to be at risk for a prolonged recovery by the team ATCs and MD, and were referred to skilled physical therapy. Results of time to MD evaluation, physical therapy evaluation, time to RTP after PT evaluation, and total time to RTP is demonstrated in Table 2. No athlete reported symptom exacerbation following clearance for RTP or full academic participation. Upon completion of the season, data was collected on the rate of re-injury following full RTP with no non-contact lower extremity injuries and one subsequent concussion occurring in the pilot cohort. It is worthwhile to note that there were also no subsequent concussions or LE injuries in any athlete in the cohort in the following football season.
DISCUSSION AND CLINICAL SIGNIFICANCE
The focus of this commentary is to describe the development and potential benefit of standardized physical therapy in a RTP protocol. It is hoped that the incorporation of sensitive sideline measures and evidence-based prognostication into sideline assessment aided in initiating early-onset individualized PT and improved outcomes. It has been demonstrated that athletes who receive clinical evaluation prior to eight days post injury have a decreased RTP time, with all PT evaluations occurring before this cut-off.33 The introduction of more sensitive diagnostic tools may have also positively affected outcomes by mitigating misdiagnosis, as athletes who suffer a concussion but continue to play are 2.2 times more likely to have a prolonged recovery.49
More notably, all athletes in the pilot group initiated sub-symptom aerobic cardiovascular exercise prior to 10 days post-injury, began vestibular rehabilitation prior to 10 days post-injury, and received individualized vestibular and cervical rehabilitation and sport-related dual-task practice. Prior to discharge, neuromotor performance was assessed and compared to baseline CTSIB data, and athletes completed a PT-supervised readiness-to-return assessment to ensure adequate vestibular function, sensory integration and motor planning, and exertional tolerance. As mentioned above, no athlete had symptom exacerbation after full academic clearance or RTP.
Prior to the implementation of the protocol described, no records were kept of athletes’ concussion histories or RTP data by the university’s athletic department or athletic training staff; therefore, it is difficult to assess the improvements in RTP time or re-injury rate compared to previous seasons. However, there are several studies previously reported on concussion recovery in collegiate athletes. A 2021 study by Putukian et al. that included 1,152 collegiate athletes found the average time to full RTP was 20.21 days.50 The United States Air Force Academy also analyzed the recovery of 104 male division 1 collegiate athletes, finding a mean time to full RTP of 20.47 days and an increase in time to RTP for those with the pre-existing risk factor of a previous concussion (1 = 35.9 days, ≥2 = 48.4 days).51
From 2013-2020, a large prospective cohort study was conducted analyzing concussion recovery data from 20 Ivy league and Big Ten Conference institutions.18 With data collected from 1,715 student-athletes, the median time to RTP was 14 days. The largest study conducted by the NCAA included data from 1,751 athletes across 22 academic institutions and reported that the median time to RTP was 12.8 days.52 All studies did report that a history of previous concussions was a risk for a longer recovery. However, no study reported data regarding athletes specifically referred to physical therapy. The variability of average time to RTP reported throughout the literature is noteworthy. However, despite all athletes in the cohort demonstrating risk for a prolonged recovery > 21 days and seven subjects having a history of one or more previous concussions, the average time of 17 days and median time of 15.5 days to RTP is comparable to larger studies.
Although the authors were unable to compare re-injury risk to previous seasons, it has been established that the risk of sustaining a subsequent concussion is significant, with an odds ratio of 3.73.53 Athletes may also be more likely to sustain a LE injury post-concussion, as Lynall et al. found that athletes are 1.97 times more likely to sustain an acute lower extremity injury within one-year post-concussion.3 As stated previously, there were no non-contact lower extremity injuries in this cohort. One athlete did sustain an ankle injury via another player falling onto the planted lower extremity. Due to the nature of the injury, it is difficult to assess if impaired motor control or motor planning increased the risk of this particular injury. One athlete sustained a second concussion later in the season; however, no athlete in the cohort sustained a subsequent concussion or LE injury in the following season, which is notable considering the increased risk of injury.
One athlete was referred to speech-language pathology as the primary symptoms/impairments were of the cognitive/fatigue clinical profile and the athlete demonstrated migraines with partial-school work that persisted longer than 14 days. However, no athlete reported symptom exacerbation with full academic participation.
There are several limitations to this pilot study. Firstly, there was no data collected regarding return-to-play, return-to-learn, or rate of post-concussion lower extremity injury prior to the implementation of this protocol, so a direct comparison of pre-implementation and post-implementation statistics is not possible. It is also important to note that three athletes did not return to athletic participation due to their decisions to discontinue play or withdraw from play with the intention to transfer to another university. These athletes did participate in skilled physical therapy and completed RTL guidelines, but their RTP data was not included in the study as they did not return to athletic participation. As mentioned above, there was one athlete who sustained a subsequent concussion. This injury occurred during the last week of the season; therefore, the data from the second injury was also not included in the data analysis, as the individual was playing in his final season and not attempting to return to play. These exclusions may have affected the results.
The authors also understand that the cohort involved in the study is very small and hesitate to make major interpretations from the data obtained. However, the authors believe that the results from the first year of protocol implementation and the discussion in this commentary have clinical relevance for collegiate athletic programs nationwide. This study continues to collect data on time to RTP and rate of re-injury with standardized referrals to skilled PT. Additional, larger, and multi-center studies are still needed to further examine the outcomes of standardized physical therapy in RTP protocols at the collegiate level.
CONCLUSION
The rate of sport-related concussions in collegiate football continues to increase and emerging data demonstrate that there are neurocognitive and neuromotor impairments that can persist past RTP. Professional organizations including the NATA, the NCAA, and the CISG have developed recent policies and recommendations to aid in SRC management; however, there continues to be a lack of continuity between policies amongst collegiate football programs and divisions of competition. Physical therapy management has been proven efficacious in reducing prolonged impairment post-concussion and increasing time to RTP, but the inclusion of physical therapy in RTP protocols is not yet common practice.
This commentary presents a RTP protocol that incorporates standardized physical therapy referral and management. Despite all athletes in the associated pilot study cohort having risk factors for prolonged recovery, the results of this protocol as measured in time to RTP are comparable to larger studies. It is also notable that there was only one subsequent concussion and zero non-contact LE injuries in the cohort after two seasons of play. The authors hesitate to make major interpretations from this study due to the small sample size; however, this commentary demonstrates that the incorporation of standardized PT in RTP is feasible and may be efficacious for athletes at risk for a protracted recovery following SRC.
Conflict of interest
All authors disclose no conflict of interests.