- Department of Neurosurgery, Tokushima University, Tokushima, Japan.
- Department of Clinical Neuroscience, Tokushima University, Tokushima, Japan.
- Department of Neurosurgery, Tokushima Red Cross Hospital, Tokushima, Japan.
Correspondence Address:
Yasuhisa Kanematsu, Department of Neurosurgery, Tokushima University, Tokushima, Japan.
DOI:10.25259/SNI_533_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Yasuhisa Kanematsu1, Kenji Shimada1, Yoshiteru Tada1, Masaaki Korai1, Takeshi Miyamoto1, Shu Sogabe1, Izumi Yamaguchi1, Yoko Yamamoto1, Nobuaki Yamamoto2, Yuki Yamamoto2, Koichi Satoh3, Yasushi Takagi1. Coil embolization with overlapping horizontal low-profile stents to treat a giant thrombosed fetal posterior cerebral artery aneurysm using contralateral approach through anterior communicating artery: Case report. 12-Jul-2021;12:347
How to cite this URL: Yasuhisa Kanematsu1, Kenji Shimada1, Yoshiteru Tada1, Masaaki Korai1, Takeshi Miyamoto1, Shu Sogabe1, Izumi Yamaguchi1, Yoko Yamamoto1, Nobuaki Yamamoto2, Yuki Yamamoto2, Koichi Satoh3, Yasushi Takagi1. Coil embolization with overlapping horizontal low-profile stents to treat a giant thrombosed fetal posterior cerebral artery aneurysm using contralateral approach through anterior communicating artery: Case report. 12-Jul-2021;12:347. Available from: https://surgicalneurologyint.com/surgicalint-articles/10961/
Abstract
Background: The treatment of internal carotid artery (ICA) – posterior communicating artery aneurysms (ICPC aneurysms) is challenging when a fetal posterior cerebral artery (PCA) arises from the saccular neck. This complex angioarchitecture renders endovascular approaches difficult. Giant thrombosed IC-PC aneurysms are also hard to treat by endovascular coiling because its flow-diversion effect is insufficient.
Case Description: We report the first case of a ruptured giant thrombosed IC-PC aneurysm associated with a fetal PCA that was successfully treated by coil embolization with retrograde overlap horizontal stenting using low-profile stents introduced through the contralateral ICA. The aneurysm was completely occluded and follow-up MRI scans demonstrated the reduction of the aneurysmal size.
Conclusion: Our technique is advantageous because low-profile stents can be used to treat lesions not accessible with flow-diverter stents due their presence in complex angioarchitectures, and overlap stenting may have flow-diversion effects that can result in the complete occlusion of giant thrombosed aneurysms.
Keywords: Contralateral approach, Fetal posterior cerebral artery, Giant thrombosed aneurysm, Low-profile stent, Overlap stent
INTRODUCTION
When the origin of the fetal posterior cerebral artery (PCA) is at the saccular neck of an internal carotid artery (ICA) – posterior communicating artery (PcomA) aneurysm (IC-PC aneurysms), treatment by surgical and endovascular means poses challenges.[
Although endovascular coil embolization of giant thrombosed aneurysms yielded unsatisfactory periprocedural and long-term results,[
We report the first successful treatment of a ruptured giant thrombosed IC-PC aneurysm associated with the fetal PCA. We used coil embolization and retrograde overlap horizontal stenting with a low-profile stent through the contralateral ICA. The aneurysm was completely occluded and follow-up MRI confirmed a reduction in the aneurysmal size.
CASE PRESENTATION
A 54-year-old man suffered sudden-onset headache. A brain CT scan performed at the time of hospital admission revealed subarachnoid hemorrhage in the basal cistern and a round high-density area on the inner side of the left temporal lobe. The World Federation of Neurosurgical Societies grade was 1; the Fisher scale was 3 [
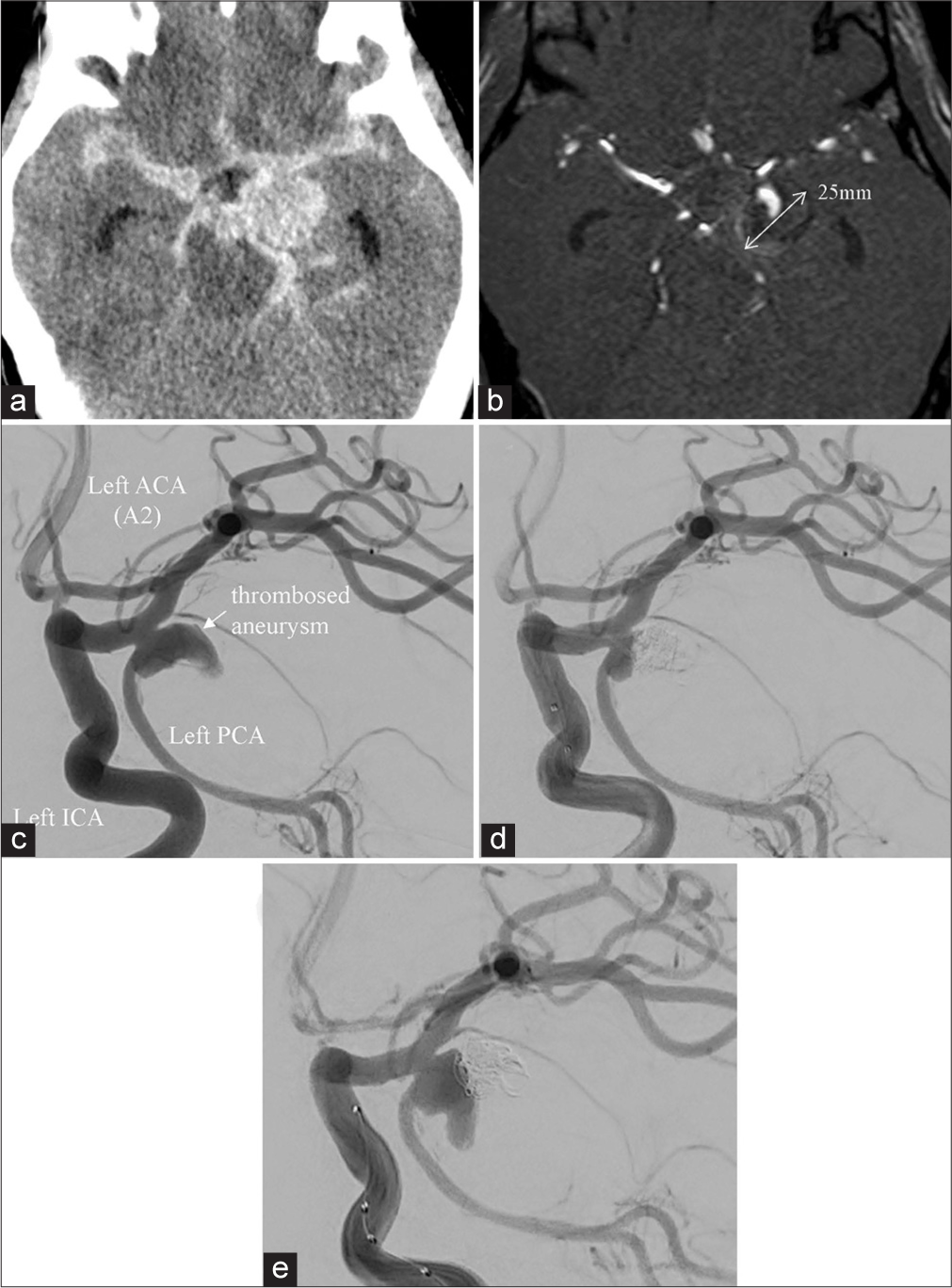
Figure 1:
(a) Brain CT scan obtained at the time of admission revealed subarachnoid hemorrhage in the basal cistern (Fisher group 3) and a round high-density area on the inner side of the left temporal lobe. (b) MRI showed a giant thrombosed aneurysm (largest diameter 25 mm). (c) Conventional angiography revealed that the aneurysm was located at the bifurcation of the left ICA and PcomA. The PcomA was fetal-type and originated at the saccular aneurysm neck. (d) Simple coil embolization was performed on the day after the insult. (e) Conventional angiography 1 month after simple coil embolization revealed coil migration into the aneurysmal thrombus and recanalization. ACA: Anterior cerebral artery, ICA: Internal carotid artery, PCA: Posterior cerebral artery, PcomA: Posterior communicating artery.
One day after the insult he underwent simple coil embolization of the aneurysmal sac [
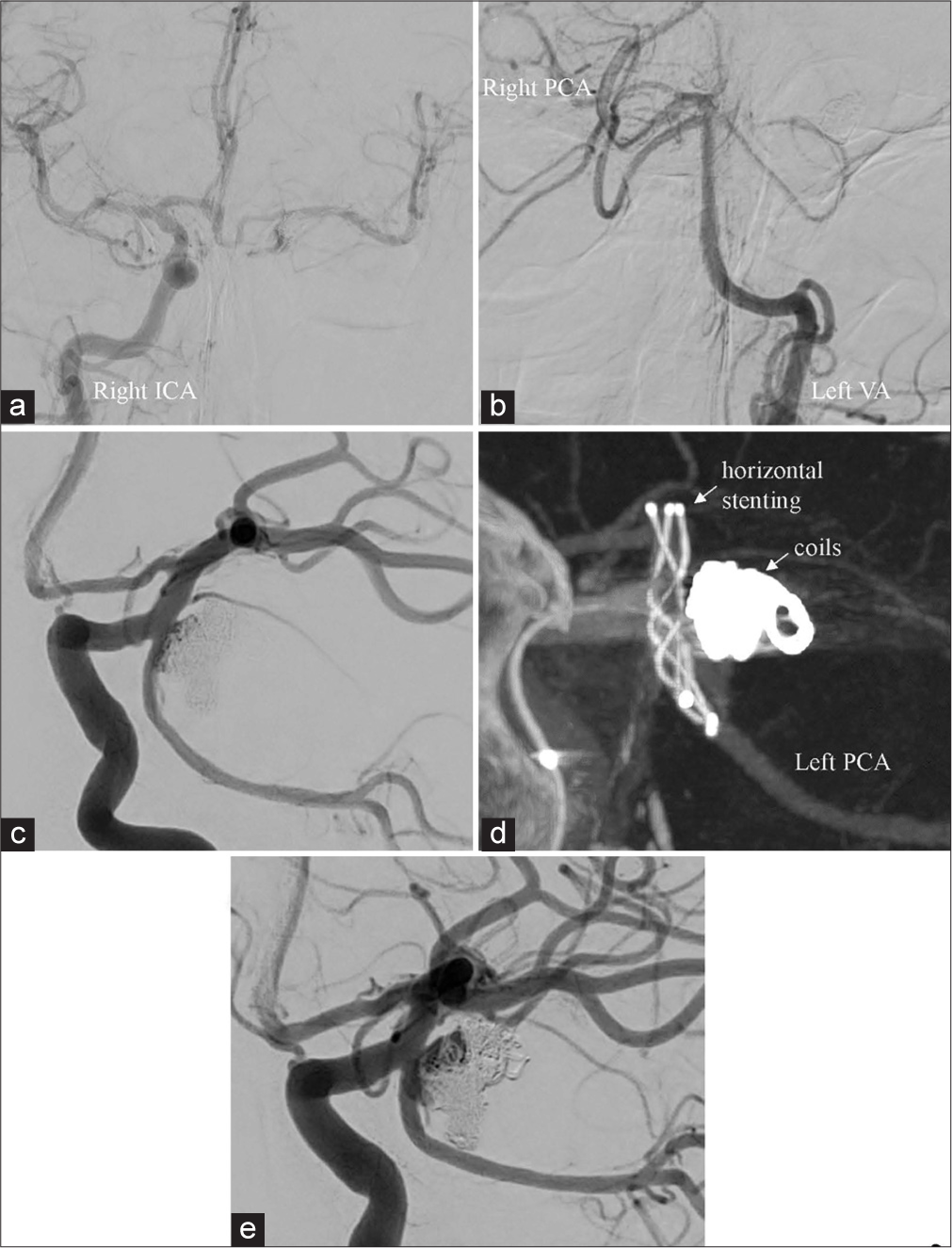
Figure 2:
(a and b) Balloon test occlusion indicated sufficient collateral flow from the contralateral ICA through the AcomA but no flow from the left VA due to the absence of the P1 segment of the left PCA. (c) Angiography after stent-assisted coil embolization indicated nearly complete occlusion and PCA patency. (d) Vaso CT revealed horizontal stenting (3.5 × 18 mm LVIS Jr. stent) from the left PCA to the left terminal ICA. (e) Follow-up angiograph obtained 2 months after the second embolization revealed coil migration into the aneurysmal thrombus and recanalization. AcomA: Anterior communicating artery, VA: Vertebral artery, PCA: Posterior cerebral artery, ICA: Internal carotid artery.
A 6-Fr arterial sheath was inserted from the right femoral artery and a 6-Fr guide catheter (Fubuki, Asahi INTEC, Aichi, Japan) was placed in the petrous portion of the right ICA. Then, we advanced a 3.2-Fr intermediate catheter (TACTICS, Technocrat Corp., Aichi, JAPAN) over an Excelsior SL-10 microcatheter (Stryker, Kalamazoo, MI, USA) using a micro-guidewire (ASAHI CHIKAI, Asahi INTEC, Aichi, Japan) and positioned TACTICS in the A1 portion of the right anterior cerebral artery. The Excelsior SL-10 microcatheter was then routed into the left terminal ICA across the AcomA, and introduced into the left PCA through the left PcomA. Subsequently, we inserted a 6-Fr guide catheter (ENVOY, Cordis, FL, USA) into the petrous portion of the left ICA. Then, the Excelsior SL-10 microcatheter was inserted into the aneurysmal sac. A 3.5 × 18 mm LVIS Jr. stent (Microvention-Terumo, CA, USA) was retrogradely deployed from the left PCA to the left terminal ICA and under horizontal stent coverage, coils were inserted through the jailed microcatheter.
These procedures achieved almost complete aneurysmal occlusion; flow in the PCA was preserved [
At that point, we considered overlap stenting across the aneurysmal neck and additional coil embolization would result in flow diversion and a reduction in the intraaneurysmal flow and in shear stress on the aneurysmal wall. The microcatheter was introduced into the left PCA from the contralateral ICA across the AcomA using the same device as before. Insertion of the microcatheter (Headway Duo, Microvention-Terumo) into the aneurysmal sac using the trans-cell approach resulted in complete coil occlusion. After coil embolization, a 3.5 × 18 mm LVIS Jr. stent that overlapped the first stent was deployed in the same manner as the first stent [
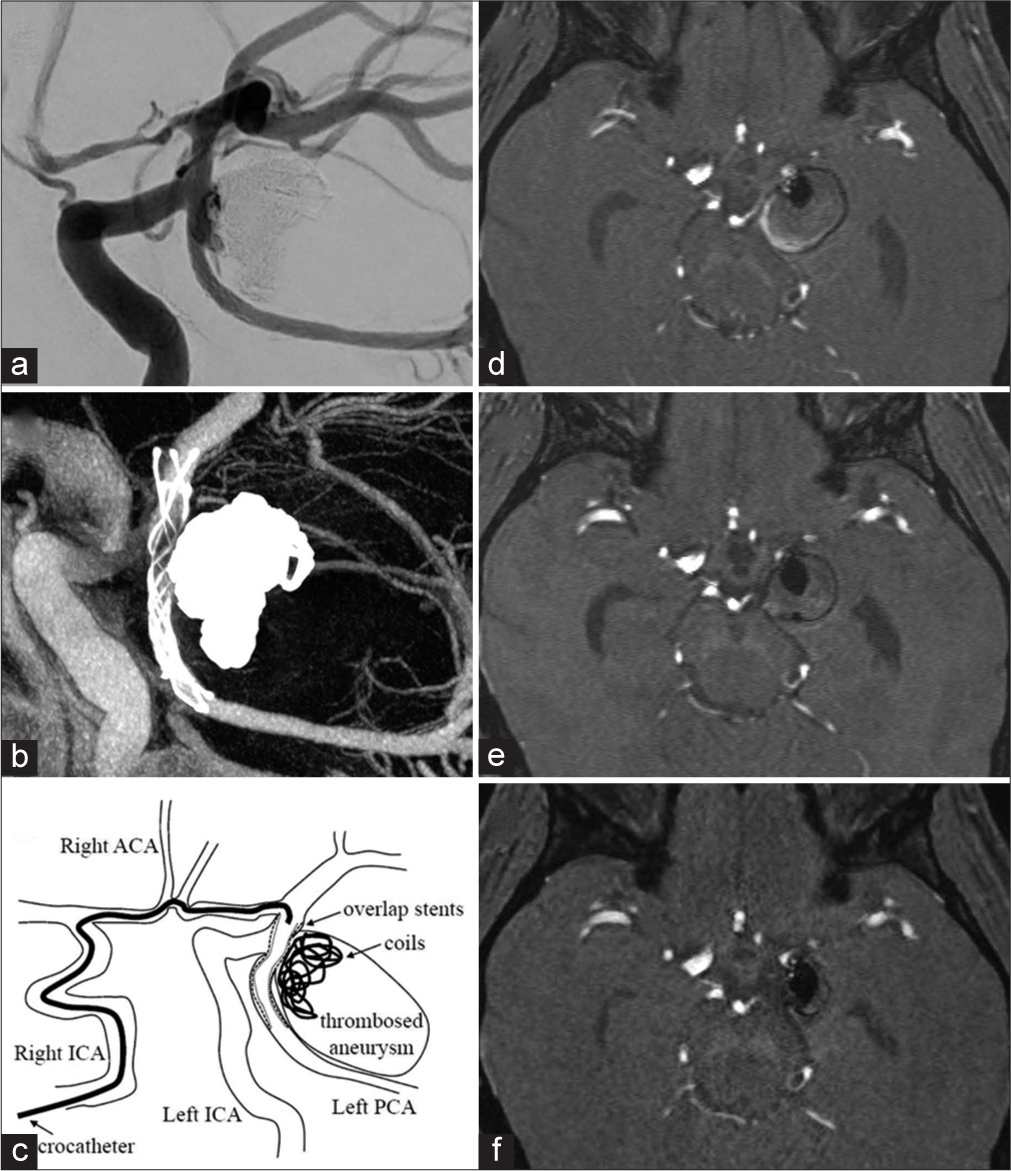
Figure 3:
(a) Angiography after coil embolization with overlap stenting indicated complete occlusion and PCA patency. (b) Vaso CT revealed deployment of a 3.5 × 18 mm LVIS Jr. stent that overlapped the first stent. (c) The schema for overlap horizontal stenting using contralateral approach via anterior communicating artery. (d) Follow-up MRI performed 1 month after stent-assisted second coil embolization revealed no marked change in the aneurysmal size. (e) Follow-up MRI performed 1 month after the third coil embolization with overlap stenting revealed a gradual decrease in the aneurysm size (19 mm). (f) Follow-up MRI performed 6 months after the third embolization showed a further decrease in the size of the aneurysm (14 mm). ACA: Anterior cerebral artery, ICA: Internal carotid artery, PCA: Posterior cerebral artery.
DISCUSSION
The long-term results of coil embolization of giant thrombosed aneurysms have been unsatisfactory; coils became buried in the thrombus of giant aneurysms due to blood flow pressure and the reported recanalization rate was 30–78%.[
Flow diverter stents promote endothelialization on the surface of the device across the aneurysmal neck and they completely block blood flow into the aneurysm, especially side-wall aneurysms.[
To promote aneurysmal thrombosis for complete occlusion, only the aneurysmal sac needs to be excluded from the intracranial circulation. IC-PC aneurysms have been addressed by ICA stenting only, ICA-PcomA stenting, pure PcomA stenting, horizontal stenting, and Y stenting.[
Stent kinking and deformity are encountered less often after horizontal than Y stenting. For horizontal stenting, the stent-delivery microcatheter must be inserted retrogradely from the contralateral to the ipsilateral ICA across the AcomA; its arrival is at the ipsilateral PComA. When the stent-delivery microcatheter is pushed from the vertebrobasilar artery to the ipsilateral ICA across the PcomA, the stent arrives at the ipsilateral terminal ICA.[
Although low-profile stents are ideal for delivery through narrow and complex routes, their flow-diversion effect is lower than that of flow diverter stents. In our patient, we encountered recanalization after the first stent-assisted coil embolization. After we performed overlap stenting and coil embolization in a second procedure, the aneurysmal size decreased. We think that the flow-diversion effect of overlap stenting contributed to the exclusion of the aneurysm from the intracranial circulation.
The LVIS Jr stent is a braided, closed-cell stent; its metal coverage rate is higher than of laser-cut stents.[
CONCLUSION
Based on our initial experience we suggest that coil embolization using retrograde overlap horizontal stenting with low-profile stents is an effective method for managing giant thrombosed IC-PC aneurysms when a fetal PCA arises from the aneurysmal saccular neck. We are following our patient closely to evaluate the long-term outcome of our treatment method.
Ethical approval
This study was approved by the Ethics Committee of our institution (No.1934-8). Prior patient or proxy informed consent for treatment was obtained. All procedures were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Becske T, Kallmes DF, Saatci I, McDougall CG, Szikora I, Lanzino G. Pipeline for uncoilable or failed aneurysms: Results from a multicenter clinical trial. Radiology. 2013. 267: 858-68
2. Cho YD, Kim KM, Lee WJ, Kang HS, Kim JE, Han HM. Retrograde stenting through the posterior cerebral artery in coil embolization of the posterior communicating artery aneurysm. Neuroradiology. 2013. 55: 733-9
3. Cho YD, Park JC, Kwon BJ, Han MH. Endovascular treatment of largely thrombosed saccular aneurysms: Follow-up results in ten patients. Neuroradiology. 2010. 52: 751-8
4. Dall’olio M, Calbucci F, Fioravanti A, Bortolotti C, Cirillo L, Princiotta C. Revascularized giant aneurysm of the anterior communicating artery after surgery and embolization, occluded by placement of a Leo baby intracranial stent. A case report. Neuroradiol J. 2013. 26: 320-6
5. Foreman PM, Salem MM, Griessenauer CJ, Dmytriw AA, Parra-Farinas C, Nicholson P. Flow diversion for treatment of partially thrombosed aneurysms: A multicenter cohort. World Neurosurg. 2020. 135: e164-73
6. Kan P, Duckworth E, Puri A, Velat G, Wakhloo A. Treatment failure of fetal posterior communicating artery aneurysms with the pipeline embolization device. J Neurointerv Surg. 2016. 8: 945-8
7. Kim SJ, Choi IS. Midterm outcome of partially thrombosed intracranial aneurysms treated with Guglielmi detachable coils. Interv Neuroradiol. 2000. 6: 13-25
8. Kwon HJ, Cho YD, Lim JW, Koh HS, Yoo DH, Kang HS. Retrograde stenting through anterior communicating artery in coil embolization of the posterior communicating artery aneurysm: Contralateral approach. J Neuroradiol. 2021. 48: 21.e1-5
9. Lawton MT, Quinones-Hinojosa A, Chang EF, Yu T. Thrombotic intracranial aneurysms: Classification scheme and management strategies in 68 patients. Neurosurgery. 2005. 56: 441-54
10. Machi P, Costalat V, Lobotesis K, Ruiz C, Cheikh YB, Eker O. LEO baby stent use following balloon-assisted coiling: Single-and dual-stent technique-immediate and midterm results of 29 consecutive patients. AJNR Am J Neuroradiol. 2015. 36: 2096-103
11. Nelson PK, Lylyk P, Szikora I, Wetzel SG, Wanke I, Fiorella D. The pipeline embolization device for the intracranial treatment of aneurysms trial. AJNR Am J Neuroradiol. 2011. 32: 34-40
12. Park SY, Oh JS, Oh HJ, Yoon SN, Bae HG. Safety and efficacy of low-profile, self-expandable stents for treatment of intracranial aneurysms: Initial and midterm results-a systematic review and meta-analysis. Interv Neurol. 2017. 6: 170-82
13. Raper DM, Rutledge WC, Winkler EA, Abla AA. Y-stent technique for treatment of wide-necked posterior communicating artery aneurysm associated with fetal posterior cerebral artery: Technical report. World Neurosurg. 2020. 133: 173-7
14. Rinaldo L, Brinjikji W, Cloft H, Lanzino G, Gonzalez LF, Kan P. Effect of fetal posterior circulation on efficacy of flow diversion for treatment of posterior communicating artery aneurysms: A multi-institutional study. World Neurosurg. 2019. 127: e1232-6
15. Roy AK, Howard BM, Haussen DC, Osbun JW, Halani SH, Skukalek SL. Reduced efficacy of the pipeline embolization device in the treatment of posterior communicating region aneurysms with fetal posterior cerebral artery configuration. Neurosurgery. 2018. 82: 695-700
16. Tsang AC, Fung AM, Tsang FC, Leung GK, Lee R, Lui WM. Failure of flow diverter treatment of intracranial aneurysms related to the fetal-type posterior communicating artery. Neurointervention. 2015. 10: 60-6
17. Walcott BP, Stapleton CJ, Choudhri O, Patel AB. Flow diversion for the treatment of intracranial aneurysms. JAMA Neurol. 2016. 73: 1002-8
18. Wang C, Tian Z, Liu J, Jing L, Paliwal N, Wang S. Flow diverter effect of LVIS stent on cerebral aneurysm hemodynamics: A comparison with Enterprise stents and the Pipeline device. J Transl Med. 2016. 14: 199-208
19. Yan Z, Zheng K, Xiong Y, Lan F, Wang Y, Tan X. Intracranial complex ruptured aneurysms coiled with overlapping low-profile visualized intraluminal support stents: Another available option for complex ruptured intracranial aneurysms. World Neurosurg. 2019. 125: e22-8
20. Zada G, Breault J, Liu CY, Khalessi AA, Larsen DW, Teitelbaum GP. Internal carotid artery aneurysms occurring at the origin of fetal variant posterior cerebral arteries: Surgical and endovascular experience. Neurosurgery. 2008. 63: ONS55-61