
Compuflo®-Assisted Performance of a Maxillary Nerve Block in Horses: Evaluation of a Novel Technology as a Training Tool and Guide in Clinical Practice
*Corresponding Author(s):
Klaus Hopster#Department Of Clinical Studies New Bolton Center, University Of Pennsylvania, Kennett Square, PA, United States
Tel:+1 6104445800,
Email:khopster@vet.upenn.edu
# Equally contributed
Astrid Bienert-Zeit#
University Of Veterinarymedicine Hannover, Foundation, Hannover, Germany
Abstract
Objective: To determine the benefits of the CompuFlo®/DPS® technology (1) as a training tool for inexperienced operators learning to perform maxillary nerve blocks and (2) to evaluate potential benefits of this device for the experienced veterinarian performing a maxillary nerve block using the EFBI approach.
Animals (or sample) (1): Twenty-three equine cadaver heads and (2) forty horses for maxillary tooth extraction.
Procedure (1): In the first study phase veterinary students, lacking previous experience with maxillary nerve blocks performed a simulated EFBI maxillary nerve block twice, utilizing either solely surface landmarks or also CompuFlo®/DPS® technology for guidance. Fisher's exact tests were performed to assess the association of injection technique with outcomes. (2) For the second phase, horses admitted for maxillary tooth extraction were randomly assigned to group CompuFlo (CompuFlo®/DPS®-assisted maxillary nerve block) or group Control (solely anatomic landmark-guided EFBI). After baseline assessments, the success of maxillary nerve blocks was scored (0 to 6) and differences between groups were examined using the Kruskal-Wallis test (p < 0.05).
Results (1): Students had significantly higher success when using the CompuFlo®-assisted approachfor needle insertion (73.9%) as compared to the simple landmark-based EFBI technique (47.8%) (p = 0.033). (2) A significant increase in median scores was noticed in both groups after performing the nerve block with no significant difference between the groups.
Conclusion and clinical relevance: The CompuFlo®-device provides value as training tool for veterinary professionals learning to execute maxillary nerve blocks. Further research is warranted to determine its true benefits in equine dentistry practice.
ABBREVIATIONS
CT: Computed tomography;
DPS: Dynamic Pressure Sensing;
EFBI: Extra-periorbital Fat Body Insertion;
IV: Intravenous;
μV: Microvolt;
V: Volt;
mmHg: Millimeters mercury
INTRODUCTION
In the standing horse, many surgical procedures involving the upper teeth, paranasal sinuses, and maxilla can be performed by employing chemical restraint and desensitizing the maxillary branch of the trigeminal nerve [1]. One widely applied technique to block the maxillary nerve within the pterygopalatine fossa, often referred to as “Maxillary Foramen Block”, has first been described by Fletcher [2]. It involves a needle insertion just ventral to the zygomatic arch until the needle tip contacts the perpendicular plate of the palatine bone, followed by infiltration of local anesthetic into the space surrounding the maxillary branch of the trigeminal nerve where it enters the maxillary foramen. However, this approach has been challenged in recent years for a number of reasons. The described land marks poorly define site and depth of needle insertion, the success rate appears to be overall low [3], and the potential for serious complications including damage to surrounding vasculature and other periorbital structures as well as infection is noteworthy [2,4-6]. To minimize the risk of vascular injury, Staszyk et al. proposed a modification to the originally described technique that involves only needle insertion and local anesthetic injection into the extra-periorbital fat body but still providing a sufficient maxillary nerve block as computed tomography images after contrast medium injection in cadaver heads indicated [6]. This approach, commonly referred to as EFBI technique, can be safely performed in sedated horses and analgesia is adequate to have animals tolerate invasive dental procedures like tooth extractions [7,8].
The CompuFlo® device (Milestone Scientific, Inc.) is a novel instrument utilizing DPS® technology that has been developed for clinical applications such as dental [9-11], epidural [12,13] and intraarticular injections. In principle, it allows detection of pressure signatures specific to tissues that a needle penetrates as it is advanced towards a target site: a nerve, the epidural space, or inner articular capsule. When performing a maxillary nerve block, this technology may serve as a useful tool to indicate to the operator the moment of needle entry into the pterygopalatine fossa since no other tissue the needle has to pass (skin, masseter muscle, fascia) will display the same unique pressure profile.
One objective of this study was to determine the value of the CompuFlo®/DPS®technology as a training/guiding tool to an inexperienced operator (senior veterinary student with appropriate knowledge of anatomy but no pre-existing clinical skills in loco-regional anesthesia or dentistry) learning to perform a maxillary nerve block in equine cadavers using the EFBI approach. We hypothesized that an untrained operator will be significantly more successful in properly executing a maxillary nerve block and avoiding major complications when having access to the CompuFlo®device as a guiding instrument versus performing the block solely on the basis of anatomic landmarks. A second objective was to evaluate the potential benefit of the CompuFlo® device as a guide for the veterinarian with dental practice experience performing a maxillary nerve block (EFBI technique) under clinical conditions. We hypothesized that CompuFlo®-assisted maxillary nerve blocks would be associated with an overall higher success rate and less complications.
MATERIALS AND METHODS
Based on the two objectives of the present study experiments were conducted in two phases.
Study phase 1
Twenty-three cadaver heads from adult horses (7 Warmblood horses, 12 Thoroughbred horse, 2 Standardbred horses, 2 draft cross breed horses) were used for this part of the study after obtaining a University of Pennsylvania’s Institutional Animal Care and Use Committee approval (No. 806340). None of the horses had a known history of dental pathologies and none showed obvious external signs of abnormal skull anatomy. The heads were sectioned at the level of the atlanto-occipital junction and stored for a maximum 2 days at 5 degrees centigrade before being used in the study. For the injection they were each placed on a high table to simulate the position in a standing horse.
Twenty-three senior students in their final year of veterinary school and with no previous experience with maxillary nerve blocks volunteered to participate in the project. Informed consent was obtained from all participants. Several hours prior to participation in the study, each student received a handout illustrating the modified surface landmark approach for a maxillary nerve block (EFBI technique described by Staszyk et al.) [6] and a description of the principles of the CompuFlo®/DPS® technology to guide an operator when performing a maxillary nerve block (Appendix 1), with no additional verbal guidance provided.
Each student performed the simulated maxillary nerve block twice. On the one side of the cadaver head the student inserted the needle solely guided by surface landmarks and immediately thereafter on the contralateral side of the same cadaver head the student performed the block with CompuFlo®-assistance. The side of the cadaver head but not the sequence in which each technique was applied was randomized, i.e. every student had to perform the nerve block using the solely landmark-guided technique first. Otherwise, any experience gained by the use of the CompuFlo® device could have had an impact of how a student executed the solely surface landmark-based technique.
Needle advancement to maxillary nerve (as explained to the students): (a) Solely landmark-guided approach (according to the technique of Staszyk et al.) [6].
A Tuohy needle[1] was inserted through the skin at a site approximately 1 cm ventral to the rim of the zygomatic arch and 1-2 cm caudal to the lateral canthus of the eye. Then it was slowly advanced in perpendicular direction, penetrating the masseter muscle and eventually the innermost fascia, which could be detected by a sudden change in insertion resistance. Thereafter, the needle was pushed forward for another 1 cm towards its final location.
(b) CompuFlo®-assisted technique /CompuFlo®-device
The continuous real-time pressure sensing technology[2] (Figure 1) was introduced to measure the pressure of tissues in real-time at the orifice of a needle [9,12-14]. Using an algorithm to determine the pressure at the tip of the needle via a continuous fluid path, the instrument provides continuously real-time “exit-pressure” data from the needle tip as the needle is advanced in-situ, thereby allowing the operator to distinguish different tissue types. The system is unique in that pressure becomes a feedback loop and controller to the system, thus regulating the electro-mechanical motor, which controls flow-rate and the fluid dispensed by the system. The instrument is equipped with such a high-resolution inline pressure sensor that it can detect pressure changes with a sensitivity of 5 μV/V/mmHg, making it more sensitive than what a human hand can sense. An audible feedback and visual display of the exit-pressure is provided to direct the operator when performing the procedure. This way the operator has an objective, quantitative method to identify the tissue type or space the needle is entering by its unique pressure signature.
Figure 1: CompuFlo® Instrument (courtesy of Milestone Scientific Inc. Livingston, NJ, USA)
During the experiments the CompuFlo®instrument was used with a flow rate of 0.05 mL/sec. Once it sensed the characteristic pressure signature corresponding to the medial fascia of the masseter muscle (increase in pressure), the needle was pushed through this fascia to subsequently enter the periorbital fat body within the pterygopalatine fossa (noticeable by a sudden decrease in pressure). The needle was then advanced one more cm to reach its final position and subsequently disconnected from the tubing of the CompuFlo®device.
Simulated maxillary foramen nerve block: After placing the needle into what has been appreciated by the students as target site, the students injected 0.25 mL radiographic contrast medium[3] through the needle. Thereafter (within 1 to 2 hours after injection) a Computed Tomography (CT) scan of the head was performed, and the location of the radio contrast medium spread was evaluated. Images were analyzed by a single observer (CHI) who was unaware of the method used. The CT images were acquired using the CereTom[4] system. For this purpose, the cadaveric heads were placed on a table and the mobile gantry was arranged over the patient table. Fifty-five to sixty transverse scans were performed at 1.25 mm intervals and analyzed in soft-tissue mode.
An injection was defined as successful if the radio contrast medium was in direct contact with the maxillary nerve or within the periorbital fat body within the pterygopalatine fossa (Figure 2). Unsuccessful injection was defined as radiographic contrast medium not being identified within the fat tissue in the pterygopalatine fossa. Visible radiographic contrast medium spread within the vasculature (maxillary artery and its branches, deep facial vein) or obvious needle injuries to blood vessels was considered and counted as occurrence of complications. It was further recorded when the students did not feel the loss of resistance but hit the perpendicular plate of the palatine bone.
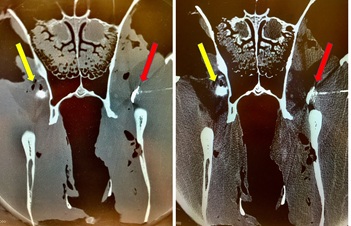 Figure 2: Computed tomography generated image (left bone mode, right soft tissue mode) of the fascia of the masseter muscle and the underlying fat body in the pterygopalatine fossa. Radiographic contrast medium (0.25 mL) was injected on both sides side of the head by inexperienced veterinary students using a solely surface landmark guided (yellow arrows) or a CompuFlo®/DPS®-assisted approach for needle insertion (red arrows).
Figure 2: Computed tomography generated image (left bone mode, right soft tissue mode) of the fascia of the masseter muscle and the underlying fat body in the pterygopalatine fossa. Radiographic contrast medium (0.25 mL) was injected on both sides side of the head by inexperienced veterinary students using a solely surface landmark guided (yellow arrows) or a CompuFlo®/DPS®-assisted approach for needle insertion (red arrows).
Study phase 2
For the second phase, 40 horses (29 Warmblood horses, 5 ponies, 6 draft cross-breed horses; 557 ± 88 kg bwt.; 5–29 years old) admitted for maxillary tooth extraction to the Clinic for Horses of the University of Veterinary Medicine Hannover, Foundation, in Hannover, Germany were enrolled in a prospective clinical study. The study involving client-owned animals had been reviewed and approved by the Animal Welfare Officer and been conducted in compliance with the current German Animal Welfare Act and after obtaining informed owner consent for all equine patients enrolled. Horses were randomly assigned to group CompuFlo (CompuFlo®-assisted maxillary nerve block) or group Control (solely anatomic landmark-based EFBI approach according to Staszyket al.,) [6].
Maxillary nerve block
All horses were sedated with IV xylazine[5] 0.3 to 0.5 mg/kg prior to performing the nerve block.
The skin puncture site for the nerve block was located as described above and desensitized by subcutaneous infiltration of 1 mL lidocaine 2%. For the maxillary nerve block a 9 cm, 20 G Tuohy needle was inserted perpendicular through the skin, masseter muscle and the fascia, which were detected by a change in insertion resistance. After entering the fat body, the needle was further inserted by approximately 1 cm.
In the CompuFlo group the device was started after puncturing the skin at the “comfort rate” level, i.e. 0.05 mL/sec. Once the CompuFlo® device displayed the characteristic pressure increase signifying needle penetration of the medial fascia of the masseter muscle, the needle was pushed through that fascia in order to enter the fat body in the pterygopalatine fossa, which was immediately detectable by an abrupt decrease in the pressure display of the instrument (see Figure 3). The needle was then advanced for another one cm towards its final location.
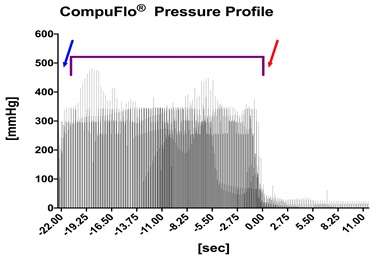
Figure 3: Detected pressure provided continuously from the needle tip as the needle is advanced through the skin (blue arrow), the masseter muscle and its medial fascia (purple bracket) and entering the fat body in the pterygopalatine fossa (time point 0, red arrow). Each bar presents the pressure value of one horse (n = 20) for that time point.
In both study groups, bupivacaine[6] 0.5% in a volume of 0.02 mL/kg body weight (according to Rieder et al.[7]) was injected once the Tuohy needle had reached its target location and been disconnected from the fluid tubing of the CompuFlo®instrument.
Before performing the nerve block as well as 15 and 30 minutes after bupivacaine administration the sensory function of the maxillary dermatome was tested as follows:
- Blunt pressure applied on the ipsilateral gingiva (right under tooth 103 or 203, respectively) using a newton meter, (Digital force/newton meter, Arbor Scientific, Ann Arbor, USA)
- Nostril clamping using forceps,
- Needle prick applied to the skin of the nostril.
The same tests were performed on the contralateral side with each horse serving as its own control. All three tests were evaluated using scores ranging from 0 to 2
2 = Complete sensory block (No response)
1 = Partial sensory block (minor nocifensive response with noticeable difference to contralateral side)
0 = No sensory block (obvious nocifensive response and no difference to the contralateral side)
The three individual scores were added up, yielding a total desensitization score ranging from 0 to 6.
[1]9 cm, 20 G; BD Tuohy Epidural Needle, Becton Dickinson and Company, Belgium
[2]CompuFlo® Instrument, Milestone Scientific Inc. Livingston, NJ, US
[3]Omnipaque®, GE Healthcare, USA
[4]Samsung Healthcare, Danvers, MA, USA
[5]Xylavet® 20 mg/mL, CP-Pharma GmbH, Burgdorf, Germany
[6]Carbostesin®, AstraZeneca, Wedel, Germany
[7]Digital force/newton meter, Arbor Scientific, Ann Arbor, USA
STATISTICAL ANALYSES
All data were analyzed using GraphPad Prism[8]software (GraphPad Software, La Jolla, CA, USA)
Study phase 1
Data were numerically coded in Excel and then Fisher's exact tests were performed to assess the association of injection technique with the binary outcomes of success/no success or complication/no complication. The level of significance was set at 5% (p < 0.05).
Study phase 2
Differences between groups and changes in desensitization scores over time within each group were examined using the Kruskal-Wallis test. The level of significance was set at 5% (p < 0.05).
[8]GraphPad Software, La Jolla, CA, USA
RESULTS
Study phase 1
Perineural injection of the maxillary nerve in the pterygopalatine fossa was attempted 46 times. Twenty-three injections were performed applying a solely surface landmark-guided technique and 23 using the CompuFlo®-assisted approach involving the novel DPS® technology. Successful perineural contrast medium deposition was achieved in 60.8% of all injections (28/46). Complications were identified in 8.7% of injections (4/46).
Contrast medium deposition into the periorbital fat body was successful in 47.8% of injections (11/23) carried out after solely surface landmark-guided needle insertion but CompuFlo®-assisted needle insertion was associated with a significantly higher success rate of 73.9% (17/23)(p = 0.033).
Complications were seen in 13.1% (3/23) of surface landmark-guided needle placements but only in 4.3% (1/23) of CompuFlo®-assisted needle insertions. However, there was no significant association between needle insertion method and overall complication rate (p = 0.084). Students encountered the perpendicular plate of the palatine bone with the tip of the needle significantly more often (p = 0.017) when performing a landmark-guided needle placement (8/23) compared to inserting the needle with CompuFlo®-guidance (2/23).
Study phase 2
Maxillary nerve blocks were successfully performed in all 40 horses. No animal developed a noticeable complication after injection of bupivacaine within the follow-up period of at least 48 hours.
There were no significant differences between scores assessed at baseline (prior sedation) with a median score of 1 in both groups. Fifteen and 30 minutes after performing the block a significant increase in median scores was noticed in both groups (Figure 4A,5 A). There was no difference between the groups at any time points.The scores on the contralateral, non-blocked side did not change over time and were not different between both groups (Figure 4B, 5 B).
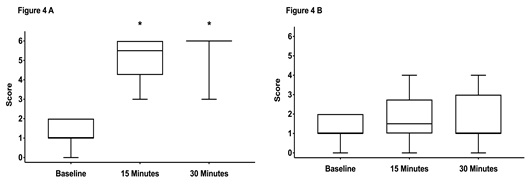
Figure 4: Box-and-whiskers plot representing the middle 50th percentile (25th to 75th) of the total sensory score of horses in group CompuFlo (n = 20).
Graph A shows the scores from the anesthetized side and graph B the scores from the non-anesthetized control side. The box represents the 75% quartile, whiskers present data min-to-max; the median is displayed as horizontal line. * indicates significant (p < 0.05) difference to baseline.
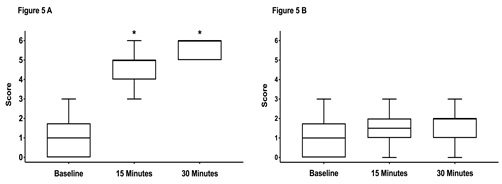
Figure 5: Box-and-whiskers plot representing the middle 50th percentile (25th to 75th) of the total sensory score of horses in group control (n = 20).
Graph A shows the scores from the anesthetized side and graph B the scores from the non-anesthetized control side. The box represents the 75% quartile, whiskers present data min-to-max; the median is displayed as horizontal line. * indicates significant (p < 0.05) difference to baseline.
DISCUSSION
The data of the current study provide evidence supporting the notion that the CompuFlo®/DPS® technology may serve as a very valuable tool when training veterinary health care professionals in the performance of maxillary nerve blocks in horses. In support of our hypothesis, upon first attempt to perform a simulated maxillary nerve block in cadaver heads senior veterinary students lacking any previous experience with this technique were significantly more successful when utilizing the CompuFlo®/DPS® technology as compared to relying solely on anatomic landmarks (73.9 vs 47.8%).This finding is in accordance with other studies reporting a success rate of less than 40% when inexperienced operators attempted to perform a maxillary nerve block using a technique solely based on surface landmarks [3], while increasing their success rate up to 65% when choosing an ultrasound guided approach for needle insertion [15,16]. Because most students had reviewed the instruction handouts immediately prior to performing the nerve block, the majority of them had no problem identifying the described landmarks and finding the proper location for skin insertion of the needle. The greatest challenge was correct identification of the fascia of the masseter muscle and the underlying fat body in the pterygopalatine fossa while inserting the needle. Consequently, the main cause for failure with the landmark only technique was the injection of contrast medium into the masseter muscle instead of the pterygopalatine fossa after penetrating the fascia with the needle. With the CompuFlo®instrument students had a technology at their disposal that helped them to unmistakably detect the initial prominent pressure rise as the needle passed the fascia of the masseter muscle and subsequently the characteristic pressure drop as the needle entered the fat body of the pterygopalatine fossa. It is likely that the trainees would have further increased their success rate, had they been given an opportunity to perform the block repeatedly.
When inexperienced veterinary students performed maxillary nerve blocks in cadaver heads during the first phase our study, the overall complication rate was 8.7% with no significant difference between the two approaches tested. This finding coincides with a report by Stauffer et al., who found both inexperienced and experienced operators execute a maxillary nerve block with greater success rate when using an ultrasound-guided approach but without significantly lowering complication rates when employing ultrasound imaging to assist them to perform the block [16]. The complication rate reported here for study phase 1 is substantially lower than that reported in a previous study in which inexperienced operators used a landmark-/ultrasound-guided technique (53.9%) but somewhat higher than that reported for experienced operators performing a maxillary nerve block in horses (3%) [3,15]. The differences in overall complication rates between our and those latter studies may be due to different methodologies, notably the use of different contrast medium volumes (0.25 mL in this study compared to 0.1 mL in the other studies), and different outcome assessments (direct hit and miss applied in this study compared to zonal system statistics in other studies).
It is noteworthy that an encounter of the perpendicular plate of the palatine bone with the tip of the needle occurred significantly more frequently when performing a solely anatomic landmark-guided versusa CompuFlo®-assisted needle placement. This is likely due to the graphic pressure display and acoustic signaling of the characteristic pressure increase when the needle is penetrating the fascia of the masseter muscle followed by the sudden pressure decline in the moment the needle tip is entering the pterygopalatine fossa (fat body). A major cause for complications associated with the landmark-based technique was the injection of contrast medium close to or into a blood vessel after the needle had hit the bone. In an alive horse, such situation may result in inadequate blockade (due to rapid uptake, binding to blood proteins, and dilution of the local anesthetic) and hematoma formation, exophthalmos, blindness and/or meningitis [6,8,15]. With reliable detection of needle penetration of the fascia and entry into the fat body in the pterygopalatine fossa a too deep advancement of the needle can be prevented and hence the risk of a blood vessel puncture in close proximity to the perpendicular plate of the palatine bone [6,17].
The findings in the second phase of our study initially appeared somewhat unexpected and contradictory to our hypothesis that the CompuFlo®-assisted approach to performing a maxillary nerve block would offer a clear benefit also in the clinical situation by increasing the success rate and reducing the risk for complications of the block. Previous studies had shown that experienced operators who can perform a solely surface landmark-guided maxillary nerve block with a success rate of up to 80% [3,18] can further increase their performance up to 100% if using ultrasound-guidance during needle insertion into the pterygopalatine fossa [15,16]. Similarly, employing continuous, quantitative, real-time, needle-tip pressure measurements using CompuFlo®/DPS® technology for epidural space identification in human patients has recently been shown to help even the most experienced anesthesiologist to improve success and reduce complication rates when performing epidural anesthesia [14].
In study phase 2, we defined a block as being successful if a desensitization score of 5 or higher was achieved after injecting the local anesthetic. In 19/20 of the horses in the CompuFlo group and 20/20 of the subjects in the Control group a desensitization score of 5 or 6 was achieved 30 minutes after drug administration, revealing a success rate of 95% and 100%, respectively, with no significant difference between groups. The veterinarian performing the maxillary nerve blocks in study phase 2 was a board-certified large animal dentist (ABZ) with over 10 years of experience in executing loco-regional anesthesia in horses for dental procedures. Insofar, the maybe unusually high success rate of this specialist executing the EFBI-technique with the conventional landmark-oriented approach and a lack of any notable complication may not come as a surprise, all the more as significantly higher success rates have been described for more experienced operators [3,18], as mentioned before. However, this does not necessarily speak against the benefit of applying the CompuFlo®/DPS® technology in the clinical setting, particularly by the less experienced equine veterinarian or a veterinarian in training.
There were a number of caveats with the design of the study that need to be addressed. A major limitation of the first study phase was the non-random sequential nature of the nerve blocks. Each student performed the blocks using a solely surface landmark orientation first, followed by the CompuFlo®-assisted approach. Each injection was performed on a different side of the head and no feedback was given to each operator between injections to minimize the effects of learning from one block to another. This methodology was based on an approach that had been taken in another comparable study [18]. Instruction material handed to the students was uniform throughout the trial and no feedback was given until after the two injections had been performed to prevent bias [18]. Also, other studies had indicated that any previous practicing of inexperienced operators without providing feedback does not have a significant effect on learning outcomes and improvements while participating in a phantom study [19,20]. Although more focused training would have provided results in favor of navigation assistance [19] we can assume that the students participating in this study did not learn from performing the first nerve block in a way that could have impacted the success rate of the second nerve block.
The clinical phase 2 of our study had two major limitations. The first limitation was the evaluation of the quality of the maxillary nerve block. Testing the local anesthetic efficacy of the EFBI-technique requires evaluation of pain-related discomfort and associated defense (nocifensive) responses in the horses. Non-specific and specific behavior patterns are described, which are associated with different conditions of pain sensation in horses [21]. Therefore, we included typical nocifensive reactions in our scoring system that can be observed when applying nociceptive stimuli to the corresponding dermatomes of the maxillary nerve on either side of the head. Responses to nociceptive stimulation were scored before and after performing the nerve block and then scores recorded after stimulation of both the ipsi- and contralateral side compared. The desensitization score used varied between 0 and 3 both during baseline recordings at the ipsi lateral side before blocking as also on the non-blocked control side indicating a moderate variability in responses among individual horses. But the significant increase in the score compared to baseline as well in comparison with the contralateral side indicated a sufficient nerve blockade in most of the horses studied. The application of contrast medium and subsequent computed tomography scanning as performed in study phase 1 would have provided significantly more precise information as to the final location of the needle tip at the time of local anesthetic administration. However, this was not an option in this population of clinical patients. Another limitation was that we tested the device only with a very experienced specialist who had refined her handling of the EFBI-technique in the many years of clinical practice that she eventually performed maxillary blocks with a very high success rate. It is likely that a less experienced equine practitioner with an average success rate of 70 to 80% [3,18] would be able to further improve his success rate using the CompuFlo®/DPS® technology. Future studies involving more operators and including less experienced clinicians are warranted to evaluate the true benefit of the CompuFlo®-assisted approach to maxillary nerve blocks in clinical practice.
CONCLUSION
Within experienced veterinary students tasked to perform in cadaver specimens a maxillary nerve block, the use of the CompuFlo®/DPS® technology proved to provide beneficial guidance to the operator when inserting the needle to its intended target location in the pterygopalatine fossa, thereby increasing the overall success rate when compared to using solely anatomic surface landmarks for guidance. Thus, the CompuFlo® instrument may serve as valuable training tool for veterinary professionals learning to execute maxillary nerve blocks. Further research is warranted to determine the true benefits of the CompuFlo®/DPS® technology in clinical equine dentistry practice.
REFERENCES
- Tremaine WH (2007) Local analgesic techniques for the equine head. Equine Vet Educ 19: 495-503.
- Fletcher BW (2004) How to perform effective equine dental nerve blocks. American Association of Equine Practitioners 233-236.
- Bardell D, Mosing I (2010) A cadaver study comparing two approaches to perform a maxillary nerve block in the horse. Equine Vet J 42: 721-725.
- Schumacher J, Perkins J (2005) Surgery of the paranasal sinuses performed with the horse standing. Clin Tech Equine Pract 4:188-194.
- Dixon PM, Hawkes C, Townsend N (2008) Complications of Equine Oral Surgery. Vet Clin North Am Equine Pract 24: 499-514.
- Staszyk C, Bienert A, Baeumer W, Feige K, Gasse H (2008) Simulation of local anaesthetic nerve block of the infraorbital nerve within the pterygopalatine fossa: anatomical landmarks defined by computed tomography. Res Vet Sci 85: 399-
- Bienert A, Rieder C, Zwick T, et al. (2011) Maxillary Nerve Block Within the Pterygopalatine Fossa - EFBI-Technique. Proceedings AAEP Focus Meeting 82-84.
- Rieder C M, Staszyk C, Hopster K, Feige K, Bienert-Zeit A (2016) Maxillary nerve block within the equine pterygopalatine fossa with different volumes: practicability, efficacy and side-effects. Pferdeheilkunde 2:132-140.
- Hochman M, Chiarello D, Hochman CB, Lopatkin R, Pergola S (1997) Computerized local anesthetic delivery vs. traditional syringe technique. Subjective Pain Response. NYS Dental J 63: 24-29.
- Hochman MN, Friedman MF, Williams WP, Hochman CB (2006) Interstitial pressure associated with dental injections: a clinical study. Quintessence Int 37: 469-476.
- Mittal M, Chopra R, Kumar A, Srivastava D (2019) Comparison of Pain Perception Using Conventional Versus Computer-Controlled Intraligamentary Local Anesthetic Injection for Extraction of Primary Molars. Anesth Prog 66: 69-76.
- Ghelber O, Gebhard RE, Vora S, Hagberg CA, Szmuk P (2008) Identification of the Epidural Space Using Pressure Measurement With the Compuflo Injection Pump-A Pilot Study. Reg Anesth Pain Med 33: 346-352.
- Gebhard RE, Moeller-Bertram T, Dobecki D, Peralta F, Pivalizza EG, et al. (2019) Objective epidural space identification using continuous real-time pressure sensing technology: A randomized controlled comparison with fluoroscopy and traditional loss of resistance. Anesth Analg129: 1319-1327.
- Hidalgo G (2019) Epidural Space Identification Using Continuous Real-Time Pressure Sensing Technology (CompuFlo®): A Report of 600 Consecutive Cases. Open J Anesth 9: 189-195.
- O'Neill H, Garcia-Pereira FL, Mohankumar PS (2014) Ultrasound-guided injection of the maxillary nerve in the horse. Equine Vet J 46: 180-184.
- Stauffer S, Cordner B, Dixon J, Witte T (2017) Maxillary nerve blocks in horses: an experimental comparison of surface landmark and ultrasound-guided techniques. Vet Anaesth Analg 44: 951-958.
- Nickel R, Schummer A, Seiferle E (1992) Lehrbuch der Anatomie der Haustiere. In: Band IV: Nervensystem, Sinnesorgane, Endokrine Drüsen. Berlin und Hamburg, Parey 307-308.
- Wilmink S, Warren-Smith CM, Roberts VL (2015) Validation of the accuracy of needle placement as used in diagnostic local analgesia of the maxillary nerve for investigation of trigeminally mediated headshaking in horses. Vet Rec 176: 148-149.
- Tielens LK, Damen LB, Lerou G, Scheffer GJ, Bruhn J (2014) Ultrasound-guided needle handling using a guidance positioning system in a phantom. Anaesthesia 69: 24-31.
- Whittaker S, Lethbridge G, Kim C (2013) An ultrasound needle insertion guide in a porcine phantom model. Anaesthesia 68: 826-829.
- Ashley FH, Waterman-Pearson AE, Whay HR (2000) Behavioural Assessment of Pain in Horses and Donkeys: Application to Clinical Practice and Future Studies. Equine Vet J 37: 565-575.
Citation: Hopster K, Bienert-Zeit A, Hopster-Iversen C, Driessen B (2020) Compuflo®-Assisted Performance of a Maxillary Nerve Block in Horses: Evaluation of a Novel Technology as a Training Tool and Guide in Clinical Practice. J Anim Res Vet Sci 4: 022.
Copyright: © 2020 Klaus Hopster#, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

