A novel sorbent based on metal–organic framework for mercury separation from human serum samples by ultrasound assisted- ionic liquid-solid phase microextraction
Vol 2, Issue 03, Pages 67-78,*** Field: Analytical Chemistry in Human
Abstract
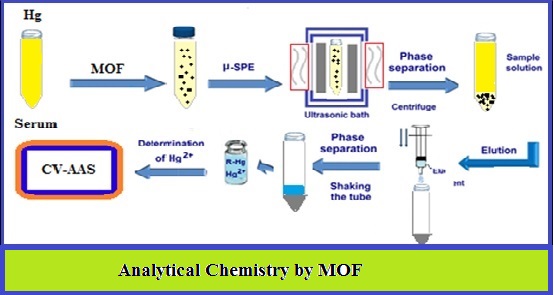
In this research, the metal–organic framework (MOF) as a solid phase was used for separation mercury [Hg (II)] inhuman serum sample by ultrasound assisted- Ionic Liquid-solid phase microextraction procedure (USA- IL-μ-SPE). Mercury extracted from serum sample by [Zn2(BDC)2(DABCO)]n as MOF at pH=7.8. Hydrophobic ionic liquid ([BMIM] [PF6]) was used as solvent trap for Hg-MOF-NC from the sample solution. The phase of Hg-MOF-NC was back extracted by 0.5 mL of HNO3 (0.2 mol L-1) and finally mercury concentration determined with cold vapor-atomic absorption spectrometry (CV-AAS) after dilution with 0.5 mL of DW. Under the optimal conditions, the linear range, limit of detection and preconcentration factor were obtained 0.02–5.5 µg L−1, 6.5 ng L−1 and 9.8 for serum samples, respectively (%RSD<5%). The validation of methodology was confirmed by standard reference materials (SRM).
References
N. Motakef-Kazemi, S.A. Shojaosadati, A. Morsali, Evaluation of the effect of nanoporous nanorods Zn2(bdc)2(dabco) dimension on ibuprofen loading and release, J. Iran. Chem. Soc. 13 (2016) 1205–1212.
S. Hajiashrafi, N. Motakef Kazemi, Preparation and evaluation of ZnO nanoparticles by thermal decomposition of MOF-5, Heliyon 5 (2019) e02152.
N. Motakef-Kazemi, S.A. Shojaosadati, A. Morsali, In situ synthesis of a drug-loaded MOF at room temperature, Micropor. Mesopor. Mat. 186 (2014) 73–79.
B. Miri, N. Motakef-Kazemi, S.A. Shojaosadati, A. Morsali, Application of a nanoporous metal organic framework based on iron carboxylate as drug delivery system, Iran. J. Pharm. Res. 17(4) (2018) 1164-1171.
S.I. Noro, S. Kitagawa, M. Kondo, K. Seki, A new methane adsorbent, porous coordination polymer, Angew Chem. Int. Ed. 39 (2000) 2082–2084.
K.S. Min, Self‐assembly and selective guest binding of three‐dimensional open‐frame work solids from a macrocyclic complex as a trifunctional metal building block, Chem. Eur. J. 7 (2001) 303–313.
M. Fujita, Y.J. Kwon, S. Washizu, K. Ogura, Preparation, clathration ability, and catalysis of a two-dimensional square network material composed of cadmium(II) and 4,4'-bipyridine, J. Am. Chem. Soc. 116 (1994) 1151–1152.
O.R. Evans, W. Lin, Crystal engineering of NLO materials based on metal−organic coordination networks, Acc. Chem. Res. 35 (2002) 511–522.
O.R. Evans, W. Lin, Crystal engineering of nonlinear optical materials based on interpenetrated diamondoid coordination networks, Chem. Mater. 13 (2001) 2705–2712.
M. Oh, C.A. Mirkin, Ion exchange as a way of controlling the chemical compositions of nano‐ and microparticles made from infinite coordination polymers, Angew Chem. Int. Ed. 45 (2006) 5492–5494.
R. Kitaura, S. Kitagawa, Y. Kubota, T.C. Kobayashi, K. Kindo, Y. Mita, A. Matsuo, M. Kobayashi, H. Chang, T.C. Ozawa, M. Suzuki, M. Sakata, M. Takata, Formation of a one-dimensional array of oxygen in a microporous metal-organic solid, Sci. 298 (2002) 2358–2361.
P. Horcajada, T. Chalati, C. Serre, B. Gillet, C. Sebrie, T. Baati, J.F. Eubank, D. Heurtaux, P. Clayette, C. Kreuz, J.S. Chang, Y.K. Hwang, V. Marsaud, P.N. Bories, L. Cynober, S. Gil, G. Ferey, P. Couvreur, R. Gref, Porous metal-organic-framework nanoscale carriers as a potential platform for drug delivery and imaging, Nat. Mater. 9 (2010) 172–178.
B. Chen, L. Wang, F. Zapata, G. Qian, A luminescent microporous metal−organic framework for the recognition and sensing of anions, J. Am. Chem. Soc. 130 (2008) 6718–6719.
A.J. Fletcher, K.M. Thomas, M.J. Rosseinsky, Flexibility in metal-organic framework materials: impact on sorption properties, J. Solid State Chem. 178 (2005) 2491–2510.
M.D. Allendorf, C.A. Bauer, R.K. Bhakta, R.J.T. Houk, Luminescent metal–organic frameworks, Chem. Soc. Rev. 38 (2009) 1330-1352.
S.M. Humphrey, T.J. Angliss, M. Aransay, D. Cave, L.A. Gerrard, G.F. Weldon, P.T. Wood, Bimetallic metal-organic frameworks containing the [M2,x-pdc)2-2](M = Cu, Pd, Pt; x = 4, 5) building block–synthesis, structure, and magnetic properties, Z. Anorg. Allg. Chem. 633 (2007) 2342-2353.
C.G. Silva, A. Corma, H. García, Metal–organic frameworks as semiconductors. J. Mater. Chem. 20 (2010) 3141-3156.
M. Hunsom, K. Pruksathorn, S. Damronglerd, H. Vergnes, P. Duverneuil, Electrochemical treatment of heavy metals (Cu2+, Cr6+, Ni2+) from industrial effluent and modeling of copper reduction, Water Res. 39 (2005) 610-616.
A. El-Samrani, B. Lartiges, F. Villiéras, Chemical coagulation of combined sewer overflow: heavy metal removal and treatment optimization. Water Res. 42 (2008) 951-960.
H. Ozaki, K. Sharma, W. Saktaywin, Performance of an ultra-low-pressure reverse osmosis membrane (ULPROM) for separating heavy metal: effects of interference parameters, Desalination 144 (2002) 287-294.
Z. Wang, J. Xu, Y. Hu, H. Zhao, H. Zhou, Y. Liu, Z. Lou, X. Xu, Functional nanomaterials: Study on aqueous Hg(II) adsorption by magnetic Fe3O4@SiO2-SH nanoparticles, J. Taiwan Inst. Chem. Eng. 60 (2016) 394-402.
L. Rahmanzadeh, M. Ghorbani, M. Jahanshahi, Effective removal of hexavalent mercury from aqueous solution by modified polymeric nanoadsorbent, J. Water Environ. Nanotechnol. 1 (2016) 1-8.
K. Yaghmaeian, R. Khosravi Mashizi, S. Nasseri, A.H. Mahvi, M. Alimohammadi, S. Nazmara, Removal of inorganic mercury from aquatic environments by multi walled carbon nanotubes, J. Environ. Health Sci. Eng. 13 (2015) 55.
Q.D. Qin, J. Ma, K. Liu, Adsorption of nitrobenzene from aqueous solution by MCM-41, J. Colloid Interface Sci. 315 (2007) 80–86.
L. Xie, D. Liu, H. Huang, Q. Yang, C. Zhong, Efficient capture of nitrobenzene from waste water using metal–organic frameworks, Chem. Eng. J. 246 (2014) 142–149.
D.V. Patil, P.B. Somayajulu Rallapalli, G.P. Dangi, R.J. R.S. Tayade, Somani, H.C. Bajaj, MIL-53(Al): An efficient adsorbent for the removal of nitrobenzene from aqueous solutions, Ind. Eng. Chem. Res. 50 (2011) 10516–10524.
N. T. Abdel-Ghani, G. A. El-Chaghaby, F. S. Helal, Individual and competitive adsorption of phenol and nickel onto multiwalled carbon nanotubes, J. Adv. Res. 6 (2015) 405-415.
Z. Zhaoa, X. Li, Z. Li, Adsorption equilibrium and kinetics of p-xylene on chromium-based metal organic framework MIL-101, Chem. Eng. J. 173 (2011) 150–157.
A.A. Adeyemo, I.O. Adeoye, O.S. Bello, Metal organic frameworks as adsorbents for dye adsorption: overview, prospects and future challenges, Toxicol. Environ. Chem. 94(10) (2012) 1846–1863.
M.M. Tong, D.H. Liu, Q.Y. Yang, S. Devautour-Vinot, G. Maurin, C.L. Zhong, Influence of framework metal ions on the dye capture behavior of MIL-100 (Fe, Cr) MOF type solids, J. Mater. Chem. A 1 (2013) 8534–8537.
M.A. Al-Ghouti, M.A.M. Khraisheh, S.J. Allen, M.N. Ahmad, The removal of dyes from textile wastewater: a study of the physical characteristics and adsorption mechanisms of diatomaceous earth, J. Environ. Manage. 69 (2003) 229–238.
J. Galán, A. Rodríguez, J.M. Gómez, S.J. Allen, G.M. Walker, Reactive dye adsorption onto a novel mesoporous carbon, Chem. Eng. J. 219 (2013) 62–68.
A. Sayari, S. Hamoudi, Y. Yang, Applications of pore-expanded mesoporous silica: removal of heavy metal cations and organic pollutants from wastewater, Chem. Mater. 17 (2005) 212-216.
S. Babel, T.A. Kurniawan, Low-cost adsorbents for heavy metals uptake from contaminated water: a review, J. Hazard. Mater. B97 (2003) 219–243.
L. Rasuli, A.H. Mahvi, Removal of humic acid from aqueous solution using MgO nanoparticles, J. Water Chem. Tech. 38(1) (2016) 21–27.
M.R. Mehmandoust, N. Motakef-Kazemi, F. Ashouri, Nitrate adsorption from aqueous solution by metal–organic framework MOF-5, Iran. J. Sci. Technol. Trans. A-Science. 43(2) (2019) 443–449.
A. Bhatnagara, E. Kumarb, M. Sillanpää, Nitrate removal from water by nano-alumina: Characterization and sorption studies, Chem. Eng. J. 163 (2010) 317–323.
J.D. Park, W. Zheng, Human exposure and health effects of inorganic and elemental mercury, J. Prev. Med. Public Health 45 (2012) 344–352.
M.A. Bradley, B.D. Barst, N. Basu, A review of mercury bioavailability in humans and fish, Int. J. Environ. Res. Public Health 14 (2017) 169.
B.F. Azevedo, L.B. Furieri, F.M. Pecanha, G.A. Wiggers, P.F. Vassallo, M.R. Simoes, J. Fiorim, P.R. Batista, M. Fioresi, L. Rossoni, I. Stefanon, M.J. Alonso, M. Salaices, D.V. Vassallo, Toxic effects of mercury on the cardiovascular and central nervous systems, J. Biomed. Biotechnol. 2012 (2012) 1-11.
K.M. Rice, E.M. Walker Jr, M. Wu, C. Gillette, E.R. Blough, Environmental mercury and its toxic effects, J. Prev. Med. Public Health 47 (2014) 74–83.
S.E. Orr, C.C. Bridges, Chronic kidney disease and exposure to nephrotoxic metals, Int. J. Mol. Sci. 18 (2017) 1039.
E. Afshar, H. Mohammadi-Manesh, H. Dashti Khavidaki, Removal of Hg (I) and Hg (II) ions from aqueous solutions, using TiO2 nanoparticles, Pollution. 3 (2017) 505-516.
K. Suresh Kumar Reddy, B. Rubahamya, A. Al Shoaibi, C. Srinivasakannan, Solid support ionic liquid (SSIL) adsorbents for mercury removal from natural gas, Int. J. Environ. Sci. Technol. 16 (2019) 1103–1110.
Copyright (c) 2019 Analytical Methods in Environmental Chemistry Journal

This work is licensed under a Creative Commons Attribution 4.0 International License.
JOURNAL PUBLISHING AGREEMENT
PLEASE PROVIDE US THE FOLLOWING INFORMATION,
Article entitled:
Corresponding author:
To be published in the journal:
Your Status
I am the sole author of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
I am one author signing on behalf of all co-authors of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
Please tick the above blanks (as appropriate), review the Journal Publishing Agreement, and then sign and date the document in black ink.
Published Journal Article: the author may share a link to the formal publication through the relevant DOI. Additionally theses and dissertations which contain embedded Published Journal Articles as part of the formal submission may be hosted publicly by the awarding institution with a link to the formal publication through the relevant DOI. Any other sharing of Published Journal Articles is by agreement with the publisher only.
Signed: ______________________________________ Name printed: ___________________________________________
Title and Company (if employer representative): _______________________Date: __________________________________














