Stimulation of nitric oxide–cGMP signaling results in vascular relaxation and increased muscle glucose uptake. We show that chronically inhibiting cGMP hydrolysis with the phosphodiesterase-5 inhibitor sildenafil improves energy balance and enhances in vivo insulin action in a mouse model of diet-induced insulin resistance. High-fat–fed mice treated with sildenafil plus l-arginine or sildenafil alone for 12 weeks had reduced weight and fat mass due to increased energy expenditure. However, uncoupling protein-1 levels were not increased in sildenafil-treated mice. Chronic treatment with sildenafil plus l-arginine or sildenafil alone increased arterial cGMP levels but did not adversely affect blood pressure or cardiac morphology. Sildenafil treatment, with or without l-arginine, resulted in lower fasting insulin and glucose levels and enhanced rates of glucose infusion, disappearance, and muscle glucose uptake during a hyperinsulinemic (4 mU · kg−1 · min−1)–euglycemic clamp in conscious mice. These effects occurred without an increase in activation of muscle insulin signaling. An acute treatment of high fat–fed mice with sildenafil plus l-arginine did not improve insulin action. These results show that phosphodiesterase-5 is a potential target for therapies aimed at preventing diet-induced energy imbalance and insulin resistance.
Insulin resistance is a significant risk factor associated with metabolic diseases, including type 2 diabetes, cardiovascular disease, and obesity (1). A hallmark of insulin resistance is an impaired ability of a physiological insulin concentration to stimulate muscle glucose uptake. The causative factors and sequence of events resulting in the development of insulin resistance remain unclear.
Several studies have shown that nitric oxide (NO) plays a key role in mediating the metabolic effects of insulin, including stimulation of muscle glucose uptake (2). Mice lacking endothelial NO synthase (eNOS), the enzyme catalyzing endothelial production of NO from l-arginine, are insulin resistant (3,4). Insulin-stimulated muscle glucose uptake is inhibited in rats treated with the NO synthase inhibitors l-NAME (NG-nitro-l-arginine methyl ester) or l-NMMA (NG-monomethyl-l-arginine) (5,6). Consistent with this, both insulin-resistant and type 2 diabetic patients exhibit impaired endothelial NO synthesis (7,8).
Endothelial NO activates guanylate cyclase in the surrounding smooth muscle to produce cGMP, resulting in smooth muscle relaxation and vasodilation. This pathway is attenuated via hydrolysis of cGMP by the cGMP-dependent phosphodiesterases, of which phosphodiesterase-5 is the predominant phosphodiesterase in vascular smooth muscle. In addition to modulating vascular tone, cGMP signaling can also regulate muscle glucose uptake. Inhibition of guanylate cyclase attenuated sodium nitroprusside-mediated stimulation of cGMP levels and 2-deoxyglucose uptake in isolated rat soleus (9) and extensor digitorum longus (10) muscles. Correspondingly, incubation of isolated rat epitochlearis muscle with a cell-permeable cGMP analog increased glucose transport (11), and blocking cGMP hydrolysis with the phosphodiesterase inhibitor zaprinast elevated cGMP levels and increased lactate release in isolated rat soleus muscles (12).
Given the role of NO-cGMP signaling in muscle metabolism, the current studies addressed the hypothesis that augmenting cGMP signaling by preventing cGMP hydrolysis would enhance in vivo insulin action in a mouse model of insulin resistance. C57BL/6J mice were placed on a high-fat diet for 12 weeks, a regimen shown to induce insulin resistance (13). To prevent cGMP hydrolysis, mice were treated with sildenafil, a highly selective (half-maximal inhibitory concentration = 3.5 nmol/l) phosphodiesterase-5 inhibitor (14). Three different protocols were designed to test the effect of phosphodiesterase-5 inhibition on insulin action in vivo. In the first protocol, mice were chronically treated during the 12-week feeding period with both the NO donor l-arginine and sildenafil to stimulate production of cGMP and inhibit cGMP degradation. In the second protocol, mice were acutely treated for 1 day with sildenafil and l-arginine after 12 weeks of high-fat feeding. In the third protocol, mice were chronically treated for 12 weeks with sildenafil alone to determine whether endogenous NO production was a limiting factor to the effects of phosphodiesterase-5 inhibition on insulin action. We show that chronic inhibition of phosphodiesterase-5 with sildenafil results in improved energy balance and enhanced insulin action in conscious high-fat–fed mice.
RESEARCH DESIGN AND METHODS
Sildenafil was isolated from Viagra tablets (Pfizer) as previously described (15) and dissolved in 0.9% saline (0.1% formic acid, pH ∼5). For the sildenafil plus l-arginine mixture, l-arginine (Sigma) was dissolved in saline containing sildenafil and adjusted to pH ∼5 with 88% formic acid. Vehicle injections consisted of 0.9% saline (0.1% formic acid, pH ∼5).
Animal models.
Animal procedures were approved by the Vanderbilt animal care and use committee. Male 5-week-old C57BL/6J mice (Jackson Laboratory) were placed on a high-fat diet (F3282; Bio-Serv) for 12 weeks. In one set of experiments (protocol 1), mice were subcutaneously injected twice daily (100 μl/injection) with sildenafil plus l-arginine (12 and 150 mg · kg−1 · day−1, respectively) or vehicle during the 12-week high-fat feeding period. This dosage of sildenafil is increased relative to the standard human dose (0.7–1 mg · kg−1 · day−1), given the higher rate of metabolism of sildenafil in the mouse (16). In another set of experiments (protocol 2), after 12 weeks on a high-fat diet, mice were subcutaneously injected with either sildenafil plus l-arginine (12 and 150 mg · kg−1 · day−1, respectively) or vehicle both 18 and 5 h before undergoing a hyperinsulinemic-euglycemic clamp. In a third set of experiments (protocol 3), mice were injected twice daily with either sildenafil (12 mg · kg−1 · day−1) or vehicle during the 12-week high-fat feeding period.
Assessment of body composition, feeding, and energy expenditure.
Body composition was determined after 10 weeks of treatment using an mq10 nuclear magnetic resonance analyzer (Bruker Optics). For food intake assessment, mice were placed in individual cages with measured amounts of food and bedding. The remaining food was weighed 48 h later, excluding fecal matter and bedding. Oxygen consumption (Vo2) was measured using an Oxymax indirect calorimetry system (Columbus Instruments) with an air flow of 0.6 l/min. After 18–24 h of adaptation, Vo2 was measured in individual mice for 1 min at 15-min intervals over a 24-h period. Energy expenditure was calculated as: (3.815 + 1.232 × RER) × Vo2, where RER is the respiratory exchange ratio (17). Ambulatory activity was estimated by the number of infrared beams broken in both X and Y directions.
Echocardiography and blood pressure measurements.
At 1 week before surgical preparation for hyperinsulinemic-euglycemic clamps, echocardiograms were performed on resting conscious mice using a 15-MHz transducer (Sonos 5500 system; Agilent) as previously described (18,19). Systolic blood pressure and pulse rate were measured in conscious mice using tail cuff plethysmography (20).
Hyperinsulinemic-euglycemic clamps.
Mice were catheterized at least 5 days before experimentation, as described previously (21). Hyperinsulinemic-euglycemic clamps were performed on mice fasted for 5 h before insulin infusion (21). A 5-μCi bolus of [3-3H]glucose was given at t = −90 min before insulin infusion, followed by a 0.05 μCi/min infusion for 90 min. All blood samples were obtained via an arterial catheter (21). Basal glucose specific activity was determined from blood samples at t = −15 and −5 min. Fasting insulin levels were determined from blood samples taken at t = −5 min. The clamp was begun at t = 0 min with a continuous infusion of human insulin (4 mU · kg−1 · min−1, Humulin R; Eli Lilly). The [3-3H]glucose infusion was increased to 0.2 μCi/min for the remainder of the experiment. Euglycemia (∼150–160 mg/dl) was maintained by measuring blood glucose every 10 min starting at t = 0 min and infusing 50% dextrose as necessary. Mice received saline-washed erythrocytes from donors throughout the clamp (5–6 μl/min) to prevent a fall of >5% hematocrit. A 12-μCi bolus of 2[14C]deoxyglucose (2[14C]DG) was given at t = 78 min. Blood samples (80–240 μl) were taken every 10 min from t = 80–120 min and processed to determine plasma [3-3H]glucose and 2[14C]DG levels. Clamp insulin was determined at t = 100 and 120 min. At t = 120 min, mice were anesthetized with sodium pentobarbital. The soleus, gastrocnemius, superficial vastus lateralis, epididymal fat, liver, diaphragm, heart, brain, and intrascapular brown fat were excised, immediately frozen, and stored at −80°C until analyzed.
Processing of plasma and muscle samples.
Immunoreactive insulin was assayed with a double-antibody method (22). Free fatty acids (FFAs) were measured spectrophotometrically by an enzymatic colorimetric assay (NEFA C kit; Wako Chemicals). Circulating cGMP levels were determined from pooled plasma samples using a cGMP immunoassay kit (Sigma). After deproteinization with barium hydroxide [Ba(OH)2, 0.3 N] and zinc sulfate (ZnSO4, 0.3 N), plasma [3-3H]glucose and 2[14C]DG radioactivity was determined by liquid scintillation counting (Packard TRI-CARB 2900TR) with Ultima Gold (Packard) as scintillant. Muscle samples were weighed and homogenized in 0.5% perchloric acid. Homogenates were centrifuged and neutralized with KOH. One aliquot was counted directly to determine 2[14C]DG and 2[14C]DG-6-phosphate (2[14C]DGP) radioactivity. A second aliquot was treated with Ba(OH)2 and ZnSO4 to remove 2[14C]DGP and any tracer incorporated into glycogen, and then it was counted to determine 2[14C]DG radioactivity. 2[14C]DGP is the difference between the two aliquots. In all experiments, the accumulation of 2[14C]DGP was normalized to tissue weight. Capillary density was determined in 5-μm sections of paraffin-embedded gastrocnemius muscles as previously described (23).
Isolation of whole-cell and mitochondrial extracts.
Muscle whole-cell extracts were obtained from gastrocnemius muscles (20–40 mg) homogenized in 10 μl/mg tissue M-PER lysis buffer (Pierce) supplemented with protease (Pierce) and phosphatase (Sigma) inhibitor cocktails. Homogenates were centrifuged (30 min, 4,500g), pellets were discarded, and supernatants were retained for protein determination. Mitochondrial extracts were obtained from intrascapular brown fat (80–100 mg) using a mitochondrial extraction kit (Imgenex). Protein content was determined using a bicinchoninic acid protein assay kit (Bio-Rad).
Immunoprecipitation and immunoblotting.
For immunoprecipitation, 250 μg of whole-cell extract was incubated overnight at 4°C with 3 μg anti–phospho(Tyr612)–insulin receptor substrate (IRS)-1 antibody. Then, 20 μl Protein G PLUS-Agarose beads (Santa Cruz) was added and incubated for 1 h at 4°C. Beads were washed four times with 1 ml cold PBS and boiled in 15 μl NuPAGE LDS loading buffer. Immunoprecipitates (15 μl), whole-cell (20 μg), and mitochondrial (20 μg) extracts were separated on 10% Bis-Tris SDS-PAGE gels (Invitrogen), followed by electrophoretic transfer to polyvinylidine fluoride membranes. Primary antibodies were incubated with the membranes overnight at 4°C. Secondary antibodies were incubated at room temperature for 1 h. For Akt, membranes were exposed to chemiluminescent substrate and images taken using a VersaDoc imaging system (Bio-Rad). Densitometry was performed using Quantity One analysis software (Bio-Rad). For IRS-1/p85α and uncoupling protein-1 (UCP-1)/voltage-dependent anion channel (VDAC), the Odyssey imaging system (Li-Cor) was used for imaging and densitometry. Rabbit anti–phospho(Tyr612)–IRS-1 (1:1,000), anti–p85α phosphatidylinositol (PI) 3-kinase (1:2,000), and anti–UCP-1 (1:2,000) were from Upstate. Rabbit anti-VDAC (1:2,000) was from Sigma-Aldrich. Rabbit anti-Akt (1:1,000) and anti–phospho-Akt (1:1,000) were from Cell Signaling. Mouse anti–glyceraldehyde-3-phosphate dehydrogenase (1:2,000) was from Abcam.
Calculations.
Glucose appearance (Ra) and disappearance (Rd) were determined using Steele non–steady-state equations (24). Endogenous glucose production (endogenous Ra; mg · kg−1 · min−1) was determined by subtracting the glucose infusion rate (GIR) from total Ra. Glucose metabolic index (Rg) was calculated by the equation (25):
where 2[14C]DGPtissue is the 2[14C]DGP radioactivity in the muscle (in dpm/g), AUC 2[14C]DGplasma is the area under the plasma 2[14C]DG disappearance curve (in dpm · min · ml−1), and [arterial glucose] is the average blood glucose (in mmol/l) from t = 80–120 min of the clamp period.
Statistical analysis.
Data are the means ± SE. Differences between groups were determined by two-way ANOVA followed by Tukey's post hoc tests or by one-tailed Student's t test as appropriate. The significance level was P < 0.05.
RESULTS
Protocol 1: chronic treatment of high fat–fed mice with sildenafil plus l-arginine improves energy balance and enhances insulin action in vivo.
Male C57BL/6J mice were fed a high-fat diet and subcutaneously injected twice daily with sildenafil plus l-arginine (150 and 12 mg · kg−1 · day−1, respectively) or vehicle for 12 weeks. Chronic sildenafil plus l-arginine treatment resulted in lower weight gain compared with vehicle (Fig. 1A) because of an ∼47% reduction in fat mass (Table 1). Food intake was unaffected by chronic sildenafil plus l-arginine treatment (Table 1). However, 24-h energy expenditure was significantly increased in mice chronically treated with sildenafil plus l-arginine compared with vehicle-treated mice (Fig. 1B). Ambulatory activity was similar in both groups (Fig. 1C).
Sildenafil plus l-arginine treatment resulted in elevated cGMP levels compared with vehicle (Table 1). Pulse rate and systolic blood pressure were not affected by sildenafil plus l-arginine treatment (Table 1). Cardiac morphology was examined by echocardiography and showed increased left ventricular mass normalized for total body weight in mice treated with sildenafil plus l-arginine (Table 1). Gastrocnemius muscle capillary density was not affected by sildenafil plus l-arginine treatment (Table 1).
After a 5-h fast, mice chronically treated with sildenafil plus l-arginine had lower arterial glucose and insulin levels compared with vehicle-treated mice (Table 2). Basal circulating FFAs were higher in mice chronically treated with sildenafil plus l-arginine (Table 2). Insulin action was assessed in conscious unrestrained mice using hyperinsulinemic (4 mU · kg−1 · min−1)–euglycemic clamps (21). Arterial glucose was clamped at similar levels in both groups (Fig. 2A and Table 2). Because fasting insulin levels were lower in mice treated with sildenafil plus l-arginine, clamp insulin levels were also lower in these mice compared with vehicle-treated mice (Table 2). The GIR required to maintain euglycemia was significantly higher in mice treated with sildenafil plus l-arginine compared with vehicle-treated mice (Fig. 2B), despite lower clamp insulin levels in the former group. Thus, the insulin sensitivity index (GIR/[insulin]) was more than twofold higher in mice treated with sildenafil plus l-arginine (Table 2). Circulating FFAs were equally suppressed in both groups (Table 2).
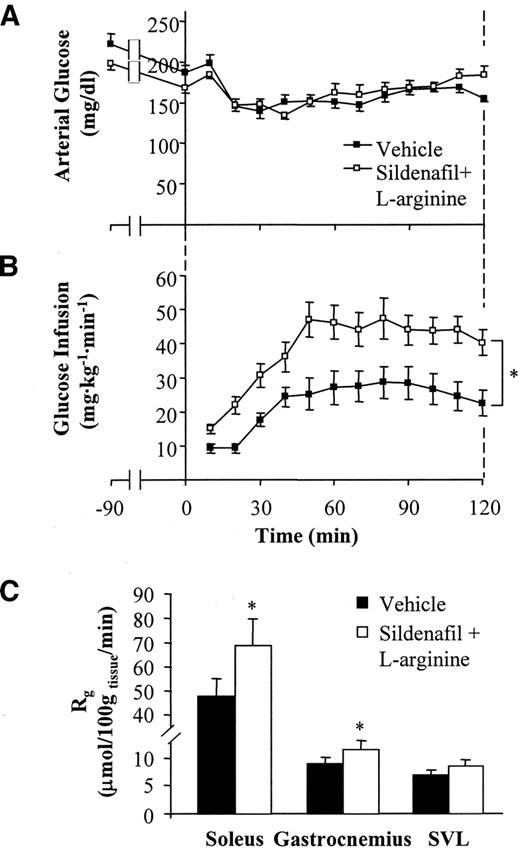
A [3-3H]glucose infusion was used to assess both basal and insulin-stimulated endogenous Ra and whole-body Rd (21). Basal endogenous Ra was not affected by chronic treatment with sildenafil plus l-arginine and was equally suppressed during the clamp in both groups (Table 2). Despite lower clamp insulin levels, Rd was enhanced in mice chronically treated with sildenafil plus l-arginine compared with vehicle-treated mice (Table 2). A bolus of 2[14C]deoxyglucose was administered to determine Rg, a measure of tissue glucose uptake. Chronic sildenafil plus l-arginine treatment resulted in enhanced Rg in soleus, gastrocnemius, and superficial vastus lateralis compared with vehicle treatment (Fig. 2C), although the effect on superficial vastus lateralis was insignificant.
Protocol 2: acute treatment with sildenafil plus l-arginine does not improve insulin action in high-fat–fed mice.
A second set of experiments was performed to determine whether acute treatment with sildenafil plus l-arginine would improve insulin action after high-fat feeding. Hyperinsulinemic-euglycemic clamps were performed on mice fed a high-fat diet for 12 weeks after subcutaneous injections of vehicle or sildenafil plus l-arginine both 18 and 5 h before clamp experiments. There was no difference in weight between mice treated with vehicle or sildenafil plus l-arginine (35.1 ± 1.3 vs. 34.3 ± 1.7 g, respectively). Arterial glucose was clamped at similar levels in both groups (151 ± 2 vs. 150 ± 3 mg/dl for vehicle vs. sildenafil plus l-arginine). The GIR required to maintain euglycemia was not different between mice treated with vehicle and sildenafil plus l-arginine (48 ± 4 vs. 45 ± 6 mg · kg−1 · min−1, respectively). There was also no difference in clamp insulin (91 ± 5 vs. 84 ± 3 μU/ml for vehicle vs. sildenafil plus l-arginine) and FFA (0.45 ± 0.11 vs. 0.37 ± 0.06 mmol/l) levels between groups. Clamp endogenous Ra (−9 ± 3 vs. −5 ± 3 mg · kg−1 · min−1 for vehicle vs. sildenafil plus l-arginine) and Rd (38 ± 2 vs. 40 ± 4) were similar in both groups. Acute treatment with sildenafil plus l-arginine also did not enhance insulin-stimulated Rg (μmol · 100 g tissue−1 · min−1) in soleus (62 ± 3 vs. 53 ± 4 for vehicle vs. sildenafil plus l-arginine), gastrocnemius (15 ± 2 vs. 12 ± 2), or superficial vastus lateralis (11 ± 1 vs. 10 ± 1).
Protocol 3: chronic inhibition of phosphodiesterase-5 with sildenafil alone improves energy balance and enhances in vivo insulin action in high fat–fed mice.
A third set of experiments was performed to determine whether chronic inhibition of phosphodiesterase-5 alone could improve insulin action in the absence of increased NO production by l-arginine. Mice were fed a high-fat diet and injected twice daily with vehicle or sildenafil (12 mg · kg−1 · day−1) for 12 weeks. Chronic sildenafil treatment resulted in decreased weight gain compared with vehicle treatment (Fig. 3A) because of an ∼30% decrease in fat mass (Table 3). This occurred despite a slight increase in daily food intake compared with vehicle treatment (Table 3). Elevated consumption was offset by an increase in 24-h energy expenditure in sildenafil-treated mice (Fig. 3B). Ambulatory activity was similar in both groups (Fig. 3C). UCP-1 levels in brown adipose tissue were insignificantly lower in sildenafil-treated mice compared with control mice (Fig. 3D).
Treatment with sildenafil alone resulted in elevated cGMP compared with control mice (Table 3). Systolic blood pressure was not affected by chronic sildenafil treatment (Table 3). Pulse rate, however, was higher in mice treated with sildenafil compared with vehicle-treated mice (Table 3). Echocardiography showed no significant effect of chronic sildenafil treatment on left ventricular mass (Table 3).
Fasting insulin levels were lower in mice chronically treated with sildenafil than in vehicle-treated mice (Table 4). Basal FFAs were similar in both groups (Table 4). Hyperinsulinemic-euglycemic clamps were performed to assess in vivo insulin action. Arterial glucose was clamped at similar levels in both groups (Fig. 4A and Table 4). The GIR required to maintain euglycemia was higher in sildenafil-treated compared with vehicle-treated mice (Fig. 4B), even though clamp insulin levels were significantly lower in the former group (Table 4). This resulted in a more than twofold higher insulin sensitivity index in sildenafil-treated compared with vehicle-treated mice (Table 4). Clamp FFAs were equally suppressed in both groups (Table 4).
Basal endogenous Ra and Rd were higher in sildenafil-treated than in vehicle-treated mice (Table 4). During the clamp, endogenous Ra was similarly suppressed in both groups, but Rd was enhanced in sildenafil-treated compared with vehicle-treated mice (Table 4). Sildenafil treatment also resulted in higher Rg in soleus, gastrocnemius, and superficial vastus lateralis compared with vehicle-treated mice (Fig. 4C), although the increase in superficial vastus lateralis was insignificant. Sildenafil treatment had no effect on binding of the p85α subunit of PI 3-kinase to tyrosine-phosphorylated IRS-1 or on Akt activation in muscle, calculated as the ratio of phosphorylated to unphosphorylated Akt (Fig. 5A and B).
DISCUSSION
The current studies demonstrate that chronic inhibition of phosphodiesterase-5 improves insulin action in a mouse model of diet-induced obesity and insulin resistance. This occurred even in the absence of an exogenous NO donor, suggesting that the endogenous supply of NO in the high fat–fed state is not limiting to the effect of sildenafil on insulin action. Chronic sildenafil treatment resulted in increased GIR and endogenous Ra, Rd, and muscle Rg during a hyperinsulinemic-euglycemic clamp, even though clamp insulin levels were lower than in vehicle-treated mice. Thus, normalizing clamp GIR by clamp insulin levels, an index of insulin sensitivity, showed a more than twofold improvement in insulin action with chronic sildenafil treatment. Chronic phosphodiesterase-5 inhibition also resulted in increased energy expenditure, suggesting that improved energy balance and weight reduction were partially responsible for the enhanced insulin action. Importantly, chronic inhibition of phosphodiesterase-5 did not result in any adverse effects on cardiac morphology or blood pressure measured in vivo, supporting human studies showing no association between long-term use of sildenafil and risk of ischemic events (26). Given the safety record of these drugs, these studies demonstrate that phosphodiesterase-5 inhibition is potentially a viable approach for the prevention of diet-induced energy imbalance and insulin resistance.
The current studies are particularly important because they demonstrate for the first time an effect on energy balance and in vivo insulin action by specifically inhibiting phosphodiesterase-5. The failure of acute sildenafil plus l-arginine treatment to enhance insulin sensitivity suggests that chronic phosphodiesterase-5 inhibition is a prophylactic measure against the development of insulin resistance. Acute administration of sildenafil plus l-arginine after a high-fat diet regimen resulted in an insignificant decrease in muscle glucose uptake. Acute administration of another phosphodiesterase-5 inhibitor, T-1032, was previously shown to inhibit muscle glucose uptake in anesthetized rats (27). Chronic sildenafil treatment had no effect on PI 3-kinase binding to tyrosine-phosphorylated IRS-1 or Akt activation in muscle. Capillary density was not significantly increased by sildenafil plus l-arginine treatment. However, an effect of chronic sildenafil treatment on capillary recruitment and increased vascular perfusion, a correlate to insulin action (28–31), cannot be ruled out. Taken together, these results demonstrate that the enhanced insulin action resulting from long-term phosphodiesterase-5 inhibition occurs by a mechanism other than insulin signaling.
One potential mechanism by which phosphodiesterase-5 inhibition may improve insulin action is prevention of endothelial dysfunction. Recent evidence supports the notion that endothelial dysfunction may be causative of insulin resistance and type 2 diabetes (32–34). Endothelial dysfunction is characterized by a decrease in NO levels, reducing cGMP production and impairing muscle glucose uptake. We used a high-fat feeding protocol previously shown to result in endothelial dysfunction, including decreased arterial vasodilation with acetylcholine and sodium nitroprusside and decreased insulin-stimulated phosphorylation of aortal eNOS (35). Thus, it is possible that preventing a decrease in cGMP levels by inhibiting phosphodiesterase-5 intervenes downstream of the site of endothelial dysfunction, resulting in improved insulin action on muscle glucose uptake.
It is also possible that the enhanced insulin action in sildenafil-treated mice resulted from an effect on the central nervous system. Sildenafil has been shown to cross the blood-brain barrier, and phosphodiesterase-5 expression has been detected in the brain (36–38). Thus, signaling through cGMP in the central nervous system may play a role in the regulation of insulin action and energy homeostasis.
The effect on insulin action in mice chronically treated with sildenafil may also be caused by improved energy balance. Mice chronically treated with sildenafil, either alone or with l-arginine, exhibited reduced weight gain and fat mass, even though food consumption was either unaffected (Table 1) or higher (Table 3) compared with vehicle-treated mice. The reduction in weight gain and fat mass was attributable to an increase in energy expenditure (Figs. 1B and 3B). This is consistent with studies demonstrating that eNOS knockout mice exhibit decreased oxygen consumption and increased weight gain (39). Thus, an impairment in NO-cGMP signaling caused by eNOS deletion results in decreased energy expenditure and increased weight gain, whereas preserving cGMP signaling, as done in the current studies, increases energy expenditure and decreases weight gain. NO signaling through cGMP has been shown to induce mitochondrial biogenesis in vitro and in vivo (39,40). Interestingly, expression of both UCP-1 and mitochondrial VDAC in brown adipose tissue was not increased in sildenafil-treated mice. It is possible that increases in cGMP by chronic sildenafil treatment may influence mitochondrial function because NO-cGMP signaling has been shown to play a role in cellular energy sensing (41,42). Furthermore, a recent epidemiological study has also shown that energy expenditure and genetic variations at the eNOS gene interact to influence the severity of glucose intolerance and the risk of developing type 2 diabetes in humans (43). It is thus clear that NO-cGMP signaling is not only a regulator of vascular tone and insulin action but is also a key player in the regulation of energy and fuel homeostasis.
Some differences were observed between mice chronically treated with sildenafil and sildenafil plus l-arginine. Daily food consumption was increased in mice treated with sildenafil, but not in mice treated with sildenafil plus l-arginine, compared with vehicle treatment. Although an anorexic effect of l-arginine has not been previously reported, it is possible that the increased nutrient load with l-arginine administration compensates for the effect of sildenafil to increase food intake. Because impaired NO signaling results in increased weight gain, it is also reasonable to suggest that stimulation of NO production by exogenous l-arginine may inhibit weight gain by suppressing food intake.
Although phosphodiesterase-5 is highly expressed in vascular smooth muscle, expression has also been detected in the heart, skeletal muscle, and brain (38,44–47). Because an increase in blood flow may enhance muscle glucose uptake by increasing delivery of substrates to skeletal muscles (28,29,48), inhibition of phosphodiesterase-5 in the arterial vasculature would be predicted to stimulate blood flow and thus muscle glucose uptake. Other studies have shown that increasing cGMP levels, either by NO donors or with the phosphodiesterase inhibitor zaprinast, results in a stimulation of glucose uptake in isolated rat skeletal muscles (9–12), suggesting that skeletal muscle cGMP levels may play a role in regulating glucose uptake. However, phosphodiesterase-5 does not represent the major cGMP hydrolyzing activity in skeletal muscle (49). Thus, the improvement in insulin action observed in the current studies is unlikely to result from inhibition of phosphodiesterase-5 in skeletal muscle. As previously mentioned, a role for central inhibition of phosphodiesterase-5 on energy and glucose metabolism is also a possibility that cannot be ruled out.
The current studies demonstrate that chronic phosphodiesterase-5 inhibition plays a role in countering the effects of high-fat diet–induced endothelial dysfunction and insulin resistance by improving energy balance and enhancing insulin action in vivo. Exogenous stimulation of the NO-cGMP pathway is not necessary because these improvements were observed even in the absence of the NO donor l-arginine. Acute stimulation of the NO-cGMP pathway with sildenafil plus l-arginine after high-fat feeding did not enhance insulin action, demonstrating that a chronic effect associated with phosphodiesterase-5 inhibition results in the prevention of diet-induced insulin resistance. Taken together, these studies highlight the interaction between energy homeostasis and insulin action and show phosphodiesterase-5 to be a key regulator of both processes.
Protocol 1: weight gain and indirect calorimetry measurements in high fat–fed C57BL/6J mice chronically treated with vehicle or sildenafil plus l-arginine. A: Body weight during a 12-week high-fat feeding period and daily treatment with vehicle or sildenafil plus l-arginine. B and C: Energy expenditure (B) and ambulatory activity (C) over a 24-h period after 10 weeks of high-fat feeding and daily treatment with either vehicle or sildenafil plus l-arginine. Data are the means ± SE for 13–14 mice per group. *P < 0.05 vs. vehicle.
Protocol 1: weight gain and indirect calorimetry measurements in high fat–fed C57BL/6J mice chronically treated with vehicle or sildenafil plus l-arginine. A: Body weight during a 12-week high-fat feeding period and daily treatment with vehicle or sildenafil plus l-arginine. B and C: Energy expenditure (B) and ambulatory activity (C) over a 24-h period after 10 weeks of high-fat feeding and daily treatment with either vehicle or sildenafil plus l-arginine. Data are the means ± SE for 13–14 mice per group. *P < 0.05 vs. vehicle.
Protocol 1: hyperinsulinemic-euglycemic clamps on 5-h–fasted, conscious, unrestrained C57BL/6J mice. A and B: Arterial glucose levels (A) and glucose infusion rates (B) during hyperinsulinemic-euglycemic clamps in mice chronically treated with either vehicle or sildenafil plus l-arginine. C: Tissue metabolic index (Rg) in soleus, gastrocnemius, and superficial vastus lateralis (SVL). Data are the means ± SE for 7–8 mice per group. *P < 0.05 vs. vehicle.
Protocol 1: hyperinsulinemic-euglycemic clamps on 5-h–fasted, conscious, unrestrained C57BL/6J mice. A and B: Arterial glucose levels (A) and glucose infusion rates (B) during hyperinsulinemic-euglycemic clamps in mice chronically treated with either vehicle or sildenafil plus l-arginine. C: Tissue metabolic index (Rg) in soleus, gastrocnemius, and superficial vastus lateralis (SVL). Data are the means ± SE for 7–8 mice per group. *P < 0.05 vs. vehicle.
Protocol 3: weight gain, indirect calorimetry measurements, and immunoblots in high fat–fed C57BL/6J mice chronically treated with vehicle or sildenafil. A: Body weight during a 12-week high-fat feeding period and daily treatment with vehicle or sildenafil. B and C: Energy expenditure (B) and ambulatory activity (C) over a 24-h period after 10 weeks of high-fat feeding and daily treatment with either vehicle or sildenafil. D: Representative image of immunoblots from brown fat mitochondrial extracts for UCP-1 and VDAC after hyperinsulinemic-euglycemic clamps. Data are the means ± SE for 8–10 mice per group. ▪, vehicle; □, sildenafil. *P < 0.05 vs. vehicle.
Protocol 3: weight gain, indirect calorimetry measurements, and immunoblots in high fat–fed C57BL/6J mice chronically treated with vehicle or sildenafil. A: Body weight during a 12-week high-fat feeding period and daily treatment with vehicle or sildenafil. B and C: Energy expenditure (B) and ambulatory activity (C) over a 24-h period after 10 weeks of high-fat feeding and daily treatment with either vehicle or sildenafil. D: Representative image of immunoblots from brown fat mitochondrial extracts for UCP-1 and VDAC after hyperinsulinemic-euglycemic clamps. Data are the means ± SE for 8–10 mice per group. ▪, vehicle; □, sildenafil. *P < 0.05 vs. vehicle.
Protocol 3: hyperinsulinemic-euglycemic clamps on 5-h–fasted, conscious, unrestrained C57BL/6J mice. A and B: Arterial glucose levels (A) and glucose infusion rates (B) during hyperinsulinemic-euglycemic clamps in mice chronically treated with either vehicle or sildenafil. C: Tissue metabolic index (Rg) in soleus, gastrocnemius, and superficial vastus lateralis (SVL). Data are the means ± SE for 7–8 mice per group. *P ≤ 0.05 vs. vehicle.
Protocol 3: hyperinsulinemic-euglycemic clamps on 5-h–fasted, conscious, unrestrained C57BL/6J mice. A and B: Arterial glucose levels (A) and glucose infusion rates (B) during hyperinsulinemic-euglycemic clamps in mice chronically treated with either vehicle or sildenafil. C: Tissue metabolic index (Rg) in soleus, gastrocnemius, and superficial vastus lateralis (SVL). Data are the means ± SE for 7–8 mice per group. *P ≤ 0.05 vs. vehicle.
Protocol 3: immunoblots in high fat–fed C57BL/6J mice chronically treated with vehicle or sildenafil. A: Representative images of immunoblots (IB) from gastrocnemius extracts for tyrosine-phosphorylated IRS-1 (p-IRS-1) and the p85α subunit of PI 3-kinase immunoprecipitated (IP) with antibody to tyrosine-phosphorylated IRS-1 after hyperinsulinemic-euglycemic clamps. Total (Akt) and phosphorylated (p-Akt) Akt after hyperinsulinemic-euglycemic clamps. B: Total p85α immunoprecipitated with tyrosine-phosphorylated IRS-1 and Akt activation, determined as the phosphorylated Akt–to–Akt ratio. Data are the means ± SE for 7–8 mice per group. ▪, vehicle; □, sildenafil.
Protocol 3: immunoblots in high fat–fed C57BL/6J mice chronically treated with vehicle or sildenafil. A: Representative images of immunoblots (IB) from gastrocnemius extracts for tyrosine-phosphorylated IRS-1 (p-IRS-1) and the p85α subunit of PI 3-kinase immunoprecipitated (IP) with antibody to tyrosine-phosphorylated IRS-1 after hyperinsulinemic-euglycemic clamps. Total (Akt) and phosphorylated (p-Akt) Akt after hyperinsulinemic-euglycemic clamps. B: Total p85α immunoprecipitated with tyrosine-phosphorylated IRS-1 and Akt activation, determined as the phosphorylated Akt–to–Akt ratio. Data are the means ± SE for 7–8 mice per group. ▪, vehicle; □, sildenafil.
Body composition, food intake, energy expenditure, and cardiovascular parameters in high fat–fed C57BL/6J mice chronically treated with vehicle or sildenafil plus l-arginine
| . | Vehicle . | Sildenafil + l-arginine . |
|---|---|---|
| n | 14 | 13 |
| Weight (g) | 32 ± 1.1 | 27.3 ± 0.6* |
| Fat (g) | 8.7 ± 1.0 | 4.6 ± 0.4* |
| Muscle (g) | 20.6 ± 0.3 | 19.9 ± 0.3* |
| Food consumption (g/day) | 3.0 ± 0.8 | 3.0 ± 0.8 |
| Capillary density (number per mm2)† | 423 ± 32 | 494 ± 29 |
| Arterial cGMP (pmol/ml)† | 0.27 ± 0.09 | 2.14 ± 0.32* |
| Pulse (beats per min) | 646 ± 10 | 632 ± 9 |
| Systolic blood pressure (mmHg) | 109 ± 3 | 108 ± 3 |
| Left ventricular mass (mg/g body wt) | 3.8 ± 3 | 5.1 ± 0.2* |
| . | Vehicle . | Sildenafil + l-arginine . |
|---|---|---|
| n | 14 | 13 |
| Weight (g) | 32 ± 1.1 | 27.3 ± 0.6* |
| Fat (g) | 8.7 ± 1.0 | 4.6 ± 0.4* |
| Muscle (g) | 20.6 ± 0.3 | 19.9 ± 0.3* |
| Food consumption (g/day) | 3.0 ± 0.8 | 3.0 ± 0.8 |
| Capillary density (number per mm2)† | 423 ± 32 | 494 ± 29 |
| Arterial cGMP (pmol/ml)† | 0.27 ± 0.09 | 2.14 ± 0.32* |
| Pulse (beats per min) | 646 ± 10 | 632 ± 9 |
| Systolic blood pressure (mmHg) | 109 ± 3 | 108 ± 3 |
| Left ventricular mass (mg/g body wt) | 3.8 ± 3 | 5.1 ± 0.2* |
Data are means ± SE.
P < 0.05 vs. vehicle;
circulating cGMP levels and capillary density were determined from plasma taken during, and gastrocnemius muscle excised after, hyperinsulinemic-euglycemic clamps.
Basal and clamp characteristics in high fat–fed C57BL/6J mice chronically treated with vehicle or sildenafil plus l-arginine
| . | Vehicle . | Sildenafil + l-arginine . |
|---|---|---|
| n | 7 | 8 |
| Arterial glucose (mg/dl) | ||
| Basal | 187 ± 9 | 168 ± 5* |
| Clamp | 163 ± 4 | 174 ± 4 |
| Insulin (μU/ml) | ||
| Basal | 42 ± 15 | 25 ± 8 |
| Clamp | 91 ± 14 | 61 ± 5* |
| Insulin sensitivity index, or GIR/[insulin] (mg · ml · [kg · min · mU]−1) | 315 ± 75 | 696 ± 93* |
| Endogenous Ra (mg · kg−1 · min−1) | ||
| Basal | 13 ± 2 | 13 ± 1 |
| Clamp | 3 ± 5 | −4 ± 3 |
| Rd (mg · kg−1 · min−1) | ||
| Basal | 13 ± 2 | 14 ± 1 |
| Clamp | 29 ± 4 | 38 ± 3* |
| FFA (mmol/l) | ||
| Basal | 1.03 ± 0.05 | 1.20 ± 0.07* |
| Clamp | 0.52 ± 0.06 | 0.46 ± 0.07 |
| . | Vehicle . | Sildenafil + l-arginine . |
|---|---|---|
| n | 7 | 8 |
| Arterial glucose (mg/dl) | ||
| Basal | 187 ± 9 | 168 ± 5* |
| Clamp | 163 ± 4 | 174 ± 4 |
| Insulin (μU/ml) | ||
| Basal | 42 ± 15 | 25 ± 8 |
| Clamp | 91 ± 14 | 61 ± 5* |
| Insulin sensitivity index, or GIR/[insulin] (mg · ml · [kg · min · mU]−1) | 315 ± 75 | 696 ± 93* |
| Endogenous Ra (mg · kg−1 · min−1) | ||
| Basal | 13 ± 2 | 13 ± 1 |
| Clamp | 3 ± 5 | −4 ± 3 |
| Rd (mg · kg−1 · min−1) | ||
| Basal | 13 ± 2 | 14 ± 1 |
| Clamp | 29 ± 4 | 38 ± 3* |
| FFA (mmol/l) | ||
| Basal | 1.03 ± 0.05 | 1.20 ± 0.07* |
| Clamp | 0.52 ± 0.06 | 0.46 ± 0.07 |
Data are means ± SE. Values are for 5-h–fasted, conscious, unrestrained mice at least 5 days after surgical catheterization.
P < 0.05 vs. vehicle.
Body composition, food consumption, energy expenditure, and cardiovascular parameters in high fat–fed C57BL/6J mice chronically treated with either vehicle or sildenafil
| . | Vehicle . | Sildenafil . |
|---|---|---|
| n | 10 | 10 |
| Weight (g) | 40.7 ± 1.6 | 36.5 ± 1.0* |
| Fat (g) | 15.7 ± 1.2 | 11.2 ± 0.7* |
| Muscle (g) | 23.3 ± 0.5 | 22.6 ± 0.6 |
| Food consumption (g/day) | 2.8 ± 0.8 | 4.2 ± 0.4* |
| Arterial cGMP (pmol/ml)† | 0.37 ± 0.09 | 1.72 ± 0.28* |
| Pulse (beats per min) | 587 ± 18 | 644 ± 12* |
| Systolic blood pressure (mmHg) | 104 ± 3 | 110 ± 3 |
| Left ventricular mass (mg/g body wt) | 4.5 ± 0.3 | 5.0 ± 0.4 |
| . | Vehicle . | Sildenafil . |
|---|---|---|
| n | 10 | 10 |
| Weight (g) | 40.7 ± 1.6 | 36.5 ± 1.0* |
| Fat (g) | 15.7 ± 1.2 | 11.2 ± 0.7* |
| Muscle (g) | 23.3 ± 0.5 | 22.6 ± 0.6 |
| Food consumption (g/day) | 2.8 ± 0.8 | 4.2 ± 0.4* |
| Arterial cGMP (pmol/ml)† | 0.37 ± 0.09 | 1.72 ± 0.28* |
| Pulse (beats per min) | 587 ± 18 | 644 ± 12* |
| Systolic blood pressure (mmHg) | 104 ± 3 | 110 ± 3 |
| Left ventricular mass (mg/g body wt) | 4.5 ± 0.3 | 5.0 ± 0.4 |
Data are means ± SE.
P < 0.05 vs. vehicle;
circulating cGMP levels were determined from plasma taken during hyperinsulinemic-euglycemic clamps.
Basal and clamp characteristics in high fat–fed C57BL/6J mice chronically treated with either vehicle or sildenafil
| . | Vehicle . | Sildenafil . |
|---|---|---|
| n | 7 | 8 |
| Arterial glucose (mg/dl) | ||
| Basal | 172 ± 18 | 150 ± 13 |
| Clamp | 156 ± 3 | 152 ± 3 |
| Insulin (μU/ml) | ||
| Basal | 50 ± 8 | 26 ± 6* |
| Clamp | 84 ± 10 | 54 ± 9* |
| Insulin sensitivity index, or GIR/[insulin] (mg · ml · [kg · min · mU]−1) | 370 ± 54 | 854 ± 101* |
| Endogenous Ra (mg · kg−1 · min−1) | ||
| Basal | 12 ± 1 | 15 ± 1* |
| Clamp | 3 ± 2 | 3 ± 1 |
| Rd (mg · kg−1 · min−1) | ||
| Basal | 12 ± 1 | 15 ± 1* |
| Clamp | 33 ± 4 | 43 ± 2* |
| FFA (mmol/l) | ||
| Basal | 1.88 ± 0.13 | 1.68 ± 0.09 |
| Clamp | 1.21 ± 0.13 | 1.02 ± 0.04 |
| . | Vehicle . | Sildenafil . |
|---|---|---|
| n | 7 | 8 |
| Arterial glucose (mg/dl) | ||
| Basal | 172 ± 18 | 150 ± 13 |
| Clamp | 156 ± 3 | 152 ± 3 |
| Insulin (μU/ml) | ||
| Basal | 50 ± 8 | 26 ± 6* |
| Clamp | 84 ± 10 | 54 ± 9* |
| Insulin sensitivity index, or GIR/[insulin] (mg · ml · [kg · min · mU]−1) | 370 ± 54 | 854 ± 101* |
| Endogenous Ra (mg · kg−1 · min−1) | ||
| Basal | 12 ± 1 | 15 ± 1* |
| Clamp | 3 ± 2 | 3 ± 1 |
| Rd (mg · kg−1 · min−1) | ||
| Basal | 12 ± 1 | 15 ± 1* |
| Clamp | 33 ± 4 | 43 ± 2* |
| FFA (mmol/l) | ||
| Basal | 1.88 ± 0.13 | 1.68 ± 0.09 |
| Clamp | 1.21 ± 0.13 | 1.02 ± 0.04 |
Data are means ± SE. Values are for 5-h–fasted, conscious, unrestrained mice at least 5 days after surgical catheterization.
P < 0.05 vs. vehicle.
Published ahead of print at http://diabetes.diabetesjournals.org on 17 January 2007. DOI: 10.2337/db06-0883.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Article Information
This work was supported by National Institutes of Health Grants U24 DK-59637 and RO1 DK-54902.
We thank Dr. Jackie Corbin and Dr. Sharron Francis for their helpful suggestions. We thank Angela Slater and Wanda Snead of the Vanderbilt Mouse Metabolic Phenotyping Center (VMMPC) Hormone Assay Core for performing the insulin assays, Gemin Ni and ZhiZhang Wang of the VMMPC Cardiovascular Pathophysiology Core for performance of echocardiography and blood pressure measurements, Dr. Lillian Nanney and Kelly Parman of the VMMPC Immunohistochemistry Core for detection of CD31, and Dr. J. Shawn Goodwin of the Vanderbilt Cell Imaging Shared Resource Core for assistance with imaging tissue sections for capillary density measurements.