Use of lightsheet imaging to assess pharmacological manipulation effects on zebrafish neural development
- Abstract number
- 1263
- Event
- European Microscopy Congress 2020
- DOI
- 10.22443/rms.emc2020.1263
- Corresponding Email
- [email protected]
- Session
- Unsure
- Authors
- Leonor Morgado (1), Dr. Anna Pezzarossa (1), Dr. Ruth Diez Del Corral (1), Dr. Davide Accardi (1)
- Affiliations
-
1. Champalimaud Centre for the Unknown
- Keywords
Lightsheet microscopy, pharmacological manipulation, zebrafish, neural development, imaging chamber, cell ablation
- Abstract text
The nervous system is responsible for the coordination of vital activities and behaviors. An enduring challenge in neural development studies is to decipher and describe how neurons are generated, differentiate and connect to create highly sophisticated functional neural networks [1]. An established approach to study these processes is to pharmacologically manipulate the neural development of model organisms and analyze the effects through fluorescence microscopy. However, none of these studies were performed in vivo for periods of at least 24 hours, due to the challenges of imaging live specimens with high time resolution over long periods [2]. Therefore, these inherently highly dynamic neuronal processes occurring over long-term periods are still poorly understood.
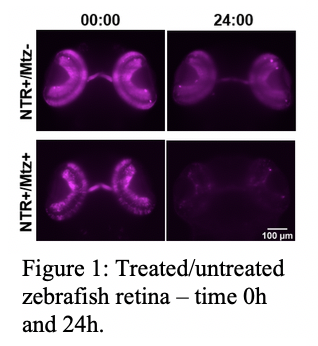
In this project we explore the potential of lightsheet microscopy to observe in vivo the effects of pharmacological manipulation on the neural development of zebrafish at the cellular level. As a proof of concept, we employed the chemical-genetic method of nitroreductase/metrodinazole-mediated ablation [3] of retina cells in 3 days post fertilisation zebrafish.
We show for the first time that it is possible to image the ablation effects with high temporal resolution (every 5 minutes) for extended periods of time (up to 24h) (Figure 1).
In addition, we developed an innovative imaging chamber for drug testing in long term in vivo imaging (Figure 2). The chamber is optically transparent, biocompatible and of small inner volume (~3 mL), which increases the efficiency of drug testing by reducing residues and costs.
Using the new chamber, we successfully replicated the ablation results previously obtained without it. The application of the chamber is not limited to neuroscience studies but could also be employed in other scientific fields that address pharmacological manipulation in living specimens.
- References
[1] E. R. Kandel, J. H. Schwartz, and T. M. Jessell, Principles of Neural Science, Fourth Edi., (McGraw-Hill Medical, 2000).
[2] J. Icha, M. Weber, J. C. Waters, and C. Norden, “Phototoxicity in live fluorescence microscopy, and how to avoid it”, BioEssays, vol. 39, no. 8, 1700003 (2017).
[3] S. Curado, D. Y. R. Stainier, and R. M. Anderson, “Nitroreductase-mediated cell/tissue ablation in zebrafish: A spatially and temporally controlled ablation method with applications in developmental and regeneration studies”, Nat. Protoc., vol. 3, no. 6, 948–954 (2008).