Department of Biochemistry and Biotechnology, Faculty of Science,
Annamalai University, Chidambaram, Tamil Nadu, India
Corresponding author email: nalininam@yahoo.com
Article Publishing History
Received: 15/12/2021
Accepted After Revision: 28/02/2022
It has been discovered that bisphenol A (BPA), an established anthropogenic xenoestrogen, is a causal factor in developing cancer, cognitive impairment, neurotoxicity, oxidative stress, and other harmful effects in humans and other species. Although there is some research into the mechanisms of BPA-induced toxicity, it is unclear whether there is a chance of amelioration through natural intervention. Zebrafish (Danio rerio) were used in this study after waterborne exposure to BPA, to assess whether carvacrol co-supplementation could reduce the destructive potential of the compound. All the chemicals and reagents utilized in the current investigations were purchased from Sigma-Aldrich, Ottochem, India. 5-7month-old zebrafish were acquired from a local fish store in Kolathur, Chennai and kept in a 50-L tank at a constant temperature of 25±2ºC.
There were no animal ethical issues involved to carry out this research. Laboratory studies were conducted to determine whether the antioxidant nature of carvacrol might protect the zebrafish brain from BPA-induced altered behavioural responses and oxidative stress. The current data demonstrates that carvacrol is effective in alleviating the altered behavioural response caused by BPA. Biochemical investigations in the zebrafish brain suggest that carvacrol may have therapeutic potential in treating oxidative stress induced by BPA. In addition, the zebrafish brain is protected by carvacrol against BPA-induced toxicity. These preliminary data suggest that carvacrol may be a helpful intervention in treating BPA-induced toxicity in zebrafish by inhibiting the reactive oxygen species production. Novel therapeutic approaches for treating BPA-induced predisposition to severe illnesses could emerge from future research on signaling cascades.
Bisphenol A, Carvacrol, Oxidative stress, Toxicity, Zebrafish.
Pratima J. B, Ragunath R, Nalini N. Carvacrol Prevents Bisphenol A-Induced Behavioral Changes And Oxidative Stress in Zebrafish Through Modulating Brain Antioxidant Defense Mechanism. Biosc.Biotech.Res.Comm. 2022;15(1).
Pratima J. B, Ragunath R, Nalini N. Carvacrol Prevents Bisphenol A-Induced Behavioral Changes And Oxidative Stress
in Zebrafish Through Modulating Brain Antioxidant Defense Mechanism. Biosc.Biotech.Res.Comm. 2022;15(1). Available from: <a href=”https://bit.ly/3IO1Z4G“>https://bit.ly/3IO1Z4G</a>
Copyright © This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY). https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provided the original author and sources are credited.
INTRODUCTION
The demand for consumer goods has increased the usage of synthetic polymers in the current manufacturing of high-quality plastic and micro-plastic materials. Their uncontrolled release into the environment poses hazard to human health in the form of the appearance of significant health problems in the future due to the release of these substances into the atmosphere. Because of its widespread use since its inception in the (1950s), bisphenol A (BPA), a synthetic compound with polymeric nature, an analogue of bisphenol (BP), is used in the production of plastics. BPA is the most frequently encountered chemical in the synthesis of epoxy and polycarbonate resins (Ansari et al. 2009; Abdalla et al. 2013). The discharge of sewage effluents and waste seepage have been identified as probable sources of BPA in surface water and groundwater.
As an additional point of reference, BPA contamination has been evident in the dust and human urine, providing further evidence of its omnipotence in the environment. The estrogenic properties of bisphenol A (BPA) make it a potent anthropogenic xenoestrogen in the endocrine-disrupting chemicals (EDC) class. These estrogenic characteristics can make it a potential endocrine disruptor (Bencan et al. 2009; Ahn et al. 2015). Bisphenol A (BPA) is lipophilic, allowing it to pass through the placenta and the blood-brain barrier and even into the breast milk (Calafat et al. 2005; Canesi et al. 2015; Ben-Jonathan et al. 2016; Barboza et al. 2020).
According to the opposing viewpoint, many studies have discovered that BPA exposure is associated with various adverse effects such as depression, cognition failure, cancer, inflammation, reproductive problems, and increased stress due to oxidation. A further concern is that increased oxidative stress has contributed to various health problems such as cardiovascular disease, ageing, cancer, and inflammation. BPA’s repercussions and oxidative stress have been widely researched in both in vitro and in vivo models focusing on organs such as the liver, colon, pancreas, and testes (Ahn et al. 2015; Barboza et al. 2020).
Nevertheless, the pathological expression of this stress in the brain has remained a mystery until now (Bindhumol et al. 2003). BPA’s increased production of reactive oxygen species (ROS) has been indicated in several earlier studies as a possible contributor to increased oxidative stress (Crain et al. 2007; Egan et al. 2009; Dong et al. 2014; Joseph et al. 2015; Eid et al. 2015; Cassar et al. 2020). As a result, further research into the potential involvement of increased BPA exposure in developing the brain stress pattern upon oxidation is required (Corrales et al. 2015; Costa et al. 2016; Das et al. 2020).
The development of significant health issues resulting from BPA discharge in water bodies near human habitation regions poses a considerable health risk to the general public and should be considered. Zebrafish (Danio rerio) is currently considered an excellent animal model for various preclinical studies because it exhibits a clear behavioural pattern and the response to different chemical interventions and stress conditions, including those induced by therapy (Ishisaka et al. 2011; Kajta et al. 2013; Chin-Chan et al. 2015; El-Horany et al. 2016). Natural intervention as a prophylactic/therapeutic strategy may be a feasible alternative to alleviate BPA-induced toxicity. Carvacrol (CVC) is a monoterpenoid phenol available in the essential oils of oregano (Origanum vulgare), thyme (Thymus vulgaris), pepperwort (Lepidium flavum) and many other plants.Several studies have demonstrated that carvacrol (CVC) has anti-inflammatory, antibacterial, antioxidant, and anticancer effects (Das et al. 2020).
As a phyto-additive in dietary supplements, carvacrol has shown to have considerable antioxidant activity and has been used successfully in animal studies to boost the antioxidant status of animals (Inadera et al. 2015; Gassman et al. 2017; Gao et al. 2018). It is most commonly used in conjugation with thymol. Many preclinical models of cancer have demonstrated that CVC has anticancer properties that are mediated by proapoptotic pathways (Kabuto et al. 2003; Heo et al. 2004; Flint et al. 2012). Research on carvacrol preventive efficacy on BPA-induced behavioural pattern changes and oxidation changes is unexplored. Therefore, in the current experiment, zebrafish was used as an in vivo model to determine the harmful effects of BPA on the brain antioxidant defense system and the alleviatory effect of carvacrol on BPA-induced changes in the brain of zebrafish (Das et al. 2020).
METHODOLOGY
All the chemicals and reagents utilized in the current investigations were purchased from Sigma-Aldrich, Ottochem, India. 5-7month-old zebrafish were acquired from a local fish store in Kolathur, Chennai and kept in a 50-L tank at a constant temperature of 25±2ºC. The 12–12 h light and dark cycle were maintained in the laboratory for zebrafish maintenance. There were no animal ethical issues involved to carry out this research. For toxicity testing and dose standardization the LC50 of BPA was analyzed, the BPA solution was prepared by dissolving it in 100% EtOH and vigorously mixing the solution.
In the end, EtOH concentration was made 0.003 percent (v/v) in all experimental groups for acute toxicity testing and BPA dose-response study. The dose-response analysis revealed that BPA caused 100 percent mortality at a concentration of 38.04 M, while the fatal concentration for BPA was estimated to be 28.28 M. The findings of this test revealed that behavioural paradigm shift occurred at a dose of 20.52 M after 96 hours, indicating that the drug was harmful. Thus, in this study, BPA dosage of 20.52 M was potent to explore the changes in zebrafish brain and nervous system.
The increasing load of BPA in the environment prompted us to investigate the effects of waterborne exposure to BPA at a substantially higher concentration than the environmentally relevant dose. Carvacrol standardization was done to determine the toxicity of carvacrol and to evaluate the LC50 and also to assess the preventive dose of carvacrol for its protective effects on BPA-induced toxicity. Following a dose-dependent investigation, the carvacrol LC50 was found to be 55.83 µM. The behavioural study revealed a quick swing in its pattern at a dose of 12.82 µM.
Because of this, carvacrol concentration of 2.96 µM was utilized in the current investigation to encase its protective effects in zebrafish. Zebrafish were grouped according to five different experimental strategies: naive, control, BPA, carvacrol, and BPA + carvacrol. Each group consisted of ten mature zebrafish, which were housed in a 15-litre experimental aquarium. According to the experimental paradigm, zebrafish from the relevant experimental groups were exposed to BPA and carvacrol for a total of 21 days (Fig.1).
In the behavioural evaluation by light and dark test it is suggested that the zebrafish have a strong preference for darker environments which is evident through the light/dark preference test (LDT). LDT was performed in the current investigation once the required experimental setup had been completed for 21 days. The adult zebrafish were moved separately and individually to the dark chamber of the apparatus after one minute of adaption in the light zone with the separating door screened between each zone. This was captured on a 5-minute video recorder after the separating gate was removed and the behavioural shifts were observed (Magno et al. 2015, Sera et al. 1999). The exploratory behaviour of zebrafish was done through the Novel Tank Test (NTT). NTT is the most frequently recommended method for analyzing zebrafish exploratory behaviour. It was discovered that zebrafish had a strong preference for spending most of their time in the bottom of the novel tank. The exploratory behaviour of zebrafish was assessed in this study (Bencan et al. 2009; Egan et al. 2009).
As soon as the studies were completed and the zebrafish behaviour had been assessed, they were sacrificed, and their brains were separated and stored at 4ºC for biochemical investigation. The zebrafish brains were used for the biochemical analysis, which was performed three-fold for each experiment (Mohanty et al. 2016). The brain samples were carefully homogenized using a glass homogenizer at 4ºC with an ice-cold RIPA buffer, then incubated at 4ºC for 25 min, and then centrifugated for 20 minutes 12,000 RPM. It was necessary to collect the supernatant, divide it and store it at -20ºC until used. Protein carbonylation determines how many carbonyls remain after oxidation (Mohanty et al. 2016).
The supernatant was separated from 10% homogenate and centrifuged at 12,000 rpm for 20 min. The supernatant was then treated with 0.5 mL 10 mM DNPH (2,4-dinitrophenylhydrazine) in 2 M HCl for 1 h at room temperature, followed by 15 minutes of vortexing every 15 minutes. To obtain the final product, 0.5 mL of 20% trichloroacetic acid was combined and centrifuged at 11,000 g for 10 minutes at 4ºC. The pellet was washed three times with 1 mL of ethanol–ethyl acetate (1:1) to remove unreactive reagents before drying. A spectrophotometer measured the concentration of carbonyls in the pellet protein at 366 nm. The samples were incubated with 2 M HCl to obtain a blank test. Carbonyl content was determined using the aliphatic hydrazone molar extinction coefficient, and results were expressed as nMole/mg carbonyl.
Lipid peroxidation test.Thymidine TBARS formation is considered a distinguishing characteristic of the peroxidation of lipids (Mohanty et al. 2017). For the most part, 100 ml of the supernatant from the brain was combined with 3.8 ml of the anti-TBARS and incubated at 95ºC for 60 minutes, after which it was centrifuged at 10,000 g for 10 minutes to remove any remaining reagent. At this point, a pink chromogen formed was evaluated at 532 nm in an ultraviolet spectrophotometer. The results were expressed in terms of moles of TBARS generated per milligram of protein.
The activity of the catalase enzyme was determined using the procedure previously described. Catalase degrades H2O2, and the amount of H2O2 degraded was measured in 15-second intervals for up to 2 minutes with a spectrophotometer at a wavelength of 240nm. nanokatal/mg protein was used to express the catalase activity, where one nano katal (nkatal) is equal to one mole of H2O2 consumed per second in the reaction mixture, and one milligram of protein (mg protein) was used to express the catalase activity measured in milligrams. Tissue glutathione (GSH) can be used to detect low levels of cytosolic oxidative stress in the tissue.
The GSH level in zebrafish brain tissue homogenate was determined in this study using the procedure that has previously been described. Phosphoric acid solution was mixed with approximately 200 mL of brain supernatant, and the solution was centrifuged at 4000 g for 15 minutes at 4ºC. A 30-minute incubation at room temperature with 5, 5-dithiobis-2-nitrobenzoic acid resulted in the generation of supernatant, which was then used to measure GSH. Once this was done, a spectrophotometric measurement at 412 nm was performed, and the amount of GSH present was expressed as micromoles per kilogram of tissue.
The glutathione reductase assay was carried out according to the previously published technique for GR activity assessment in zebrafish brain (Sarkar et al. 2014). To determine the degree of change in GSSG to GSH, a spectrophotometric measurement at 340 nm was taken, and the degree of change in GSH was calculated. When measuring glutathione reductase activity, the molar extinction coefficient of NADPH is utilized. This value is given as nmoles NADPH oxidized/min/mg protein. Using a previously developed methodology GST activity was determined (Pabst et al. 1974). To determine the amount of this enzyme (GST) present in brain tissue, it was previously necessary to observe the reaction between glutathione GSH and GST. The substrate CDNB (1-chloro-2,4-dinitrobenzene) was measured at an absorbance of 340 nm. The GSH-CDNB conjugate was used to determine the molar extinction coefficient, which was used to measure GST activity. The resultant value was reported as nanomoles of CDNB conjugate formed per minute per milligram of protein in the sample (nmole CDNB conjugate).
Our methods for calculating total SOD activity have been somewhat adjusted from the method established by (Beauchamp C 1971). The reaction mixture contained 2.9 mL 50 mM Na-phosphate buffer, 2 mM riboflavin, 10 mM EDTA, 75 mM Nitro Blue tetrazolium, 13 mM methionine, and 100 mL brain tissue aliquot which were added to a 100 mL flask. Further incubation was done at 30°C for 10 minutes to study its absorbance at 560 nm. In this work, one unit of SOD enzyme activity was defined as the amount of sample protein required to block the NBT by 50%. For statistical analysis the mean and standard deviation of the mean was used to represent all of the data. Comparing the outcomes of the different groups was done using one-way analysis of variance followed by DMRT test for comparisons between the naive and control carvacrol groups and between the BPA and the BPA + carvacrol groups. In all groups, p-value of less than 0.05 was considered statistically significant.
RESULTS AND DISCUSSION
Carvacrol co-supplementation improved the scototaxis behaviour of BPA-induced groups, which was associated with a reduction in BPA exposure: The scototaxis behaviour was significantly altered in zebrafish after its exposure to BPA in the aquatic condition, which was evident by the increase in time spent in the lighted environment than naive and control fish (Fig. 2a & b). Furthermore, latency to enter in the black zone in LDT compared to naive and control groups was very much evident after BPA exposure (Fig. 2c). Carvacrol significantly reduced the scototaxis behaviour alterations in the BPA + carvacrol group compared to the BPA group of zebrafish.
The antioxidant carvacrol helps zebrafish recover their bottom-dwelling and explorative behaviours after being supplemented with BPA: Transition to the top-zone and the time spent in the entire zone increased in the groups exposed to BPA compared to groups 1 and 2 (Fig. 3a & b). Also, when compared to groups 1 and 2, BPA-exposed group had significantly lower latency to top zone entry (Fig. 3c). The addition of carvacrol to the BPA-exposed group reduced the time spent in the top zone, the number of transitions to the entire area, and the latency to enter the top site. The findings of the current study suggest that carvacrol may protect against BPA-induced behavioural changes.
Carvacrol co-supplementation has been shown to improve the symptoms of BPA-induced oxidative stress: Compared to other groups, BPA exposure for 21 days increased LPX and protein carbonylation levels significantly (Fig. 4a & b). Catalase activity in zebrafish brains were reduced considerably after BPA exposure (Fig. 4c). The primary results of the current work reveal that the increased ROS production in the zebrafish brain resulted in the protein and lipid component breakdown compared to the control and naive groups. When used as a preventive supplement, carvacrol reduced the levels of protein carbonylation, lipid peroxidation and CAT activity in zebrafish brains exposed to BPA.
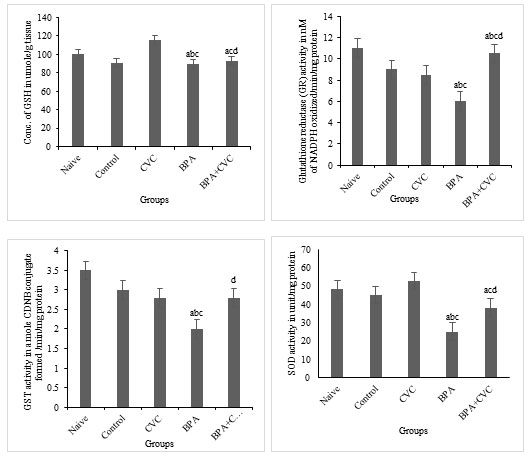
Carvacrol co-supplementation can reverse BPA-induced changes in glutathione production: The levels of BPA considerably reduced glutathione reductase activities (GR) and superoxide dismutase (SOD) activities in the brain of zebrafish (Fig. 5a, 5b, 5c and 5d) compared to naive and control zebrafish groups. According to the current study, BPA causes oxidative stress in zebrafish brains, which results in changes in antioxidant levels compared with naive and control groups. Previous studies have supported the protective role of flavonoids in restored neuronal redox equilibrium against oxidative stress. A standard concentration of carvacrol for waterborne complementation has also been proposed to deduce the function of carvacrol as a plausible mechanism of action against BPA-induced toxicity. Our research has shown that BPA has a significant influence on the antioxidant state of the zebrafish brains. As a result, carvacrol is shown to drastically reduce the ROS in the zebrafish brain by increasing antioxidants and the free radical scavenging enzymes in the cellular environment as a preventive supplement against oxidative stress induced by BPA.
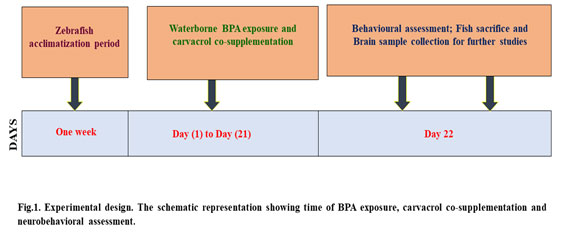
Figure 2: The light/dark test (LDT). [a] Graphs depicting the number of transitions to light zone, [b] amount of time spent, [c] and expectancy to pass through the light region after BPA exposure and carvacrol supplementation. The mean and standard deviation are used to express the values. a, b, c, d represents p< 0.05 when compared to the naïve, control, carvacrol and BPA group respectively.
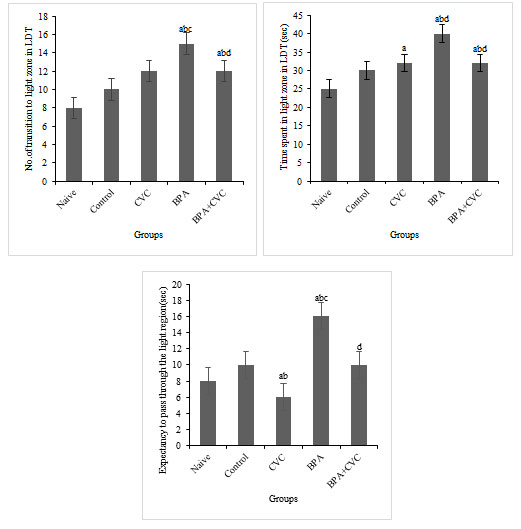
Figure 3: Novel Tank Test (NTT). [a] Graphs depicting changes in number of entries to the top zone, [b]time spent in the top zone of the tank, and [c] Expectancy to enter the top zone of the tank exposure to BPA and carvacrol. The values are given as mean ± SD. a, b, c, d represents p< 0.05 when compared to the naïve, control, carvacrol and BPA group respectively.
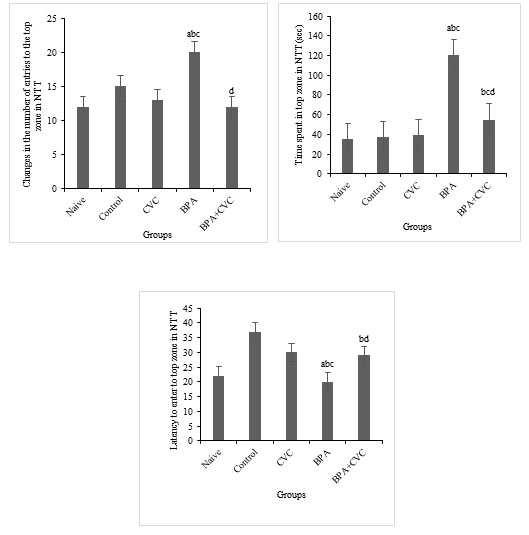
Figure 4: Assay for the parameters of oxidative stress. [a]Graphs depicting changes TBARS levels, [b] changes in the amount of protein carbonyl, and [c] changes in the catalase activity in the zebrafish brain following BPA exposure and carvacrol supplementation. The values are given as mean ± SD. a, b, c, d represents p< 0.05 when compared to the naïve, control, carvacrol and BPA group respectively.
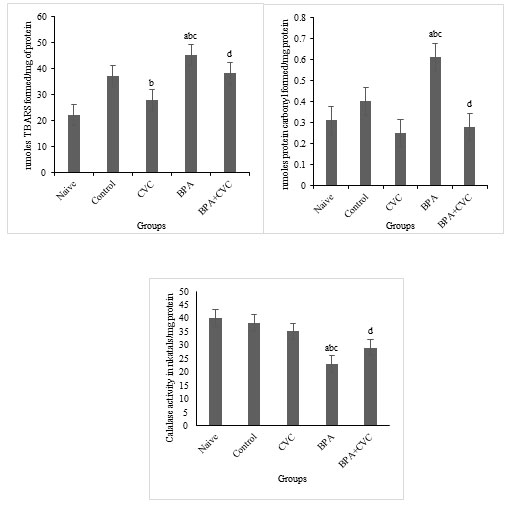
Figure 5: Enzymatic assay for free radical scavenging. [a] Graphs depicting changes in GSH concentration, [b] Glutathione reductase activity, [c] GST activity, and [d] superoxide dismutase activity in zebrafish brain after chronic BPA exposure and carvacrol supplementation. The values are given as mean ± SD. a, b, c, d represents p< 0.05 when compared to the naïve, control, carvacrol and BPA group respectively.
BPA in the environment can be a major danger to human health in developing serious health concerns. BPA is largely an anthropogenic toxin. Therefore, the current study seeks to determine the toxicity of BPA and protective effect of carvacrol on toxicity of BPA. To investigate the impact of increased BPA load on zebrafish brains, the concentration of BPA selected for this study was 20.52 M, significantly higher than the environmental relevance level in aquatic bodies. Also, the preventive efficacy of carvacrol at the concentration of 2.96 µM was studied against BPA-induced toxicity. According to the current findings, carvacrol has both ameliorative and protective effects on BPA-induced oxidative stress-mediated behavioural change and toxicity (Kelly et al. 1998; Kawato et al. 2004; Kang et al. 2006; Kang et al. 2007; Lorber et al. 2015; Kuo et al. 2017; Cassar et al. 2020). Overall, we discovered that carvacrol lowers BPA-induced oxidative stress and recovers zebrafish scototaxis and bottom-dwelling behaviour.
NTT demonstrated that co-supplementing carvacrol following waterborne BPA exposure significantly changed the bottom-dwelling habit of zebrafish when compared with the other groups. Compared to other groups, carvacrol administered group dramatically put back the altered behavioural changes generated by BPA administration, as demonstrated by a considerable decrease in the frequency of movement to the brightly lit zone and the amount of time spent in the light zone in LDT (Nishikawa et al. 2010; Nagel et al. 2013; Negri-Cesi et al. 2015; Murata et al. 2018).
By modifying scototaxis and bottom-dwelling behaviour in zebrafish and also from the preliminary data it is evident that BPA has neurotoxic potential in zebrafish (Rochester et al. 2013; Rennekamp et al. 2015). We looked at the levels of various oxidants and antioxidant enzymes in the zebrafish brain to confirm that the BPA-induced altered behavioural response is due to increased oxidative stress and see if carvacrol could protect against BPA-induced oxidative stress (Cassar et al. 2020).
When supplemented with carvacrol, it lowered ROS and lipid peroxidation in the zebrafish brain (Sangai et al. 2014). According to the findings and further investigation we found that this compound reverses the reductions in antioxidant levels and the free radical scavenging enzyme system caused by BPA, hence lowering oxidative stress in the zebrafish cerebral cortex (Winston et al. 1991; Wong et al. 2017; Xu et al. 2019). This study suggests that persistent waterborne exposure to BPA increased free radical production in zebrafish brains while simultaneously decreasing glutathione reductase function.
The zebrafish brain had low glutathione (GSH). To keep the cell environment healthy, a lively balance between glutathione synthesis and oxidation is required. Thus, glutathione reductase is vital in regulating oxidative stress. According to our findings, carvacrol can reduce the effects of BPA on glutathione reductase activity in the zebrafish brain, which has implications for the regulation of GSH levels in the zebrafish brain and other tissues (redox balance) (Xu et al. 2019; Cassar et al. 2020).
The antioxidant capacity of carvacrol is thought to be enhanced by its ability to regulate the level of cytosolic GSH, which is responsible for its protective effects. Following prior publications, our results suggest that carvacrol greatly reduces the increase in LPX in the zebrafish brain, potentially through an increase in GSH levels and superoxide dismutase activity (Eid et al. 2015). As a result, the simultaneous activation of GPX and CAT activity is essential for protection against oxidative stress, which is consistent with the involvement of SOD in superoxide radical detoxification.
Under the initial assertion, our results demonstrate that carvacrol greatly alleviated the BPA-induced downregulation of CAT activity in the zebrafish brain (Zimmers et al. 2014; Yamazaki et al. 2015). Based on our findings, carvacrol appears to be a suitable supplement for zebrafish suffering from BPA-induced oxidative stress, resulting in altered behavioural responses. Additional research on scototaxis behaviour was carried out to better understand the potential consequences of BPA exposure and establish preventive efficacy of carvacrol (Zhou et al. 2017; Barboza et al. 2020).
Our findings indicate that carvacrol co-supplementation greatly mitigated the behavioural pattern changes due to BPA exposure. To put it succinctly, human populations of developing and undeveloped countries have become indiscriminate users of plasticizers (microplastics, including BPA) in recent years. As shown in the current study, carvacrol has a better and protective effect on the behavioral changes, toxicity, and oxidative stress caused by BPA. On the whole, we found that carvacrol reduces oxidative stress due to BPA, restores zebrafish scototaxis and behaviour changes.
The NTT has revealed a significant improvement in the bottom-life habit of zebrafish when compared to naive and control groups (Serra et al. 1999; Rahal et al. 2014). The altered scototaximization behaviour in LDT caused by BPA was significantly reversed by the carvacrol co-supplementation, which is evident by the sudden downfall in the of the number of light zone transitions and time spent in light zone following administration of BPA. These findings strongly support the hypothesis that toxic potential of BPA in zebrafish by altering behaviour can be alleviated by using carvacrol (Barboza et al. 2020).
CONCLUSION
The findings of the present study suggest that the toxicity in brain caused by BPA is protected by carvacrol in zebrafish. According to the current research, increased oxidative stress caused altered behavioural responses in zebrafish. Carvacrol has been shown to effectively scavenge ROS and hydroxy radicals after prolonged aquatic exposure to BPA, it is also effective when used therapeutically. Carvacrol increases GSH levels and antioxidant enzymes in the cell, which may help protect against BPA-induced brain damage. Based on the findings of this study, carvacrol may be used to treat BPA-induced behavioural changes and oxidative stress. Novel therapeutic approaches for treating BPA-induced predisposition to severe illnesses could emerge from future research on signaling cascades.
Conflict of interests: Authors declare no conflicts of interests to disclose.
Ethical Statement: This work was done in the year 2019 and at that time as per the guidelines of CPCSEA for experimentation, there was no ethical issue involved. Also, this is a PhD research work carried out by the research scholar who registered for PhD in the year 2018. Only in the year, 2021 ethical has been added for fish experimentation. Also, with regard to this, the institutional animal ethics committee has issued a circular on ethical clearance for a fish experiment only from Feb 2022. Since the work was done before the CPSCEA decision on fish ethics. There are no ethical issues involved to carry out the research.
Data Availability Statement: The database generated and /or analysed during the current study are not publicly available due to privacy, but are available from the corresponding author on reasonable request.
REFERENCES
Abdalla, F.H., Cardoso, A.M., Pereira, L.B., et al. (2013). Neuroprotective effect of quercetin in ectoenzymes and acetylcholinesterase activities in cerebral cortex synaptosomes of cadmium-exposed rats. Mol. Cell. Biochem. 381 (September (1–2)), 1–8. https://doi.org/10.1007/s11010-013-1659-x.
Ahn, T.B. and Jeon, B.S. (2015). The role of quercetin on the survival of neuron-like PC12 cells and the expression of α-synuclein. Neural Regen. Res. 10 (July (7)), 1113–1119. https://doi.org/10.4103/1673-5374.160106.
Ansari, M.A., Abdul, H.M., Joshi, G., et al. (2009). Protective effect of quercetin in primary neurons against Abeta(1-42): relevance to Alzheimer’s disease. J. Nutr. Biochem. 20 (April (4)), 269–275. https://doi.org/10.1016/j. jnutbio.2008.03.002.
Barboza, L.G.A., Cunha, S.C., Monteiro, C., et al. (2020). Bisphenol A and its analogs in muscle and liver of fish from the North East Atlantic Ocean in relation to microplastic contamination. Exposure and risk to human consumers. J. Hazard. Mater. 393 (July), 122419. https://doi.org/10.1016/j. jhazmat.2020.122419.
Beauchamp, C and Fridovich, I., (1971). Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 44 (November (1)), 276–287. https:// doi.org/10.1016/0003-2697(71)90370-8.
Bencan, Z., Sledge, D and Levin, E.D (2009). Buspirone, chlordiazepoxide and diazepam effects in a zebrafish model of anxiety. Pharmacol. Biochem. Behav. 94 (November (1)), 75–80. https://doi.org/10.1016/j.pbb.2009.07.009.
Ben-Jonathan, N and Hugo, E.R (2016). Bisphenols come in different flavors: is “S” better than “A”? Endocrinology 157 (April (4)), 1321–1323. https://doi.org/10.1210/ en.2016-1120.
Bindhumol, V., Chitra, K.C and Mathur, P.P (2003). Bisphenol A induces reactive oxygen species generation in the liver of male rats. Toxicology 188 (June (2–3)), 117–124. https://doi.org/10.1016/s0300-483x(03)00056-8.
Calafat, A.M., Kuklenyik, Z., Reidy, J.A et al. (2005). Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ. Health Perspect. 113 (April (4)), 391–395. https://doi.org/ 10.1289/ehp.7534.
Canesi, L and Fabbri, E (2015). Environmental effects of BPA: focus on aquatic species. Dose Response 13 (July (3)). https://doi.org/10.1177/1559325815598304, 1559325815598304.
Cassar, S., Adatto, I., Freeman, J.L et al. (2020). Use of zebrafish in drug discovery toxicology. Chem. Res. Toxicol. 33 (January (1)), 95–118. https://doi.org/10.1021/ acs.chemrestox.9b00335.
Chin-Chan, M., Navarro-Yepes, J and Quintanilla-Vega, B (2015). Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and Parkinson diseases. Front. Cell. Neurosci. 9 (April), 124. https://doi.org/10.3389/fncel.2015.00124.
Corrales, J., Kristofco, L.A., Steele, W.B et al. (2015). Global assessment of bisphenol A in the environment: review and analysis of its occurrence and bioaccumulation. Dose 13 (July (3)). https://doi. org/10.1177/1559325815598308, 1559325815598308.
Costa, L.G., Garrick, J.M., Roqu`e, P.J et al. (2016). Mechanisms of neuroprotection by quercetin: counteracting oxidative stress and more. Oxid Med Cell Longev. 2016, 2986796 https://doi.org/10.1155/2016/2986796.
Crain, D.A., Eriksen, M., Iguchi, T et al. (2007). An ecological assessment of bisphenol-A: evidence from comparative biology. Reprod. Toxicol. 24 (August–September (2)), 225–239. https://doi.org/ 10.1016/j.reprotox.2007.05.008.
Das, S.K., Aparna, S and Patri, M (2020). Chronic waterborne exposure to benzo[a]pyrene induces locomotor dysfunction and development of neurodegenerative phenotypes in zebrafish. Neurosci. Lett. 716 (January), 134646. https://doi.org/10.1016/j. neulet.2019.134646.
Denny Joseph, K.M and Muralidhara (2015). Combined oral supplementation of fish oil and quercetin enhances neuroprotection in a chronic rotenone rat model: relevance to Parkinson’s disease. Neurochem. Res. 40 (May (5)), 894–905. https://doi.org/ 10.1007/s11064-015-1542-0.
Dong, Y.S., Wang, J.L., Feng, D.Y et al. (2014). Protective effect of quercetin against oxidative stress and brain edema in an experimental rat model of subarachnoid hemorrhage. Int. J. Med. Sci. 11 (3), 282–290. https://doi.org/10.7150/ijms.7634.
Egan, R.J., Bergner, C.L., Hart, P.C et al. (2009). Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav. Brain Res. 205 (December (1)), 38–44. https://doi.org/10.1016/j.bbr.2009.06.022.
Eid, J.I., Eissa, S.M and El-Ghor, A.A (2015). Bisphenol A induces oxidative stress and DNA damage in hepatic tissue of female rat offspring. J. Basic Appl. Zool. 71, 10–19. https://doi.org/10.1016/j.jobaz.2015.01.006.
El-Horany, H.E., El-Latif, R.N., ElBatsh et al. (2016). Ameliorative effect of quercetin on neurochemical and behavioral deficits in rotenone rat model of Parkinson’s disease: modulating autophagy (Quercetin on experimental Parkinson’s disease). J. Biochem. Mol. Toxicol. 30 (July (7)), 360–369. https://doi.org/10.1002/ jbt.21821.
Flint, S., Markle, T., Thompson, S et al. (2012). Bisphenol A exposure, effects, and policy: a wildlife perspective. J. Environ. Manage. 15 (August (104)), 19–34. https:// doi.org/10.1016/j.jenvman.2012.03.021.
Gao, W., Pu, L., Chen, M. et al. (2018). Glutathione homeostasis is significantly altered by quercetin via the Keap1/Nrf2 and MAPK signaling pathways in rats. J. Clin. Biochem. Nutr. 62 (January (1)), 56–62. https://doi.org/10.3164/jcbn.17-40.
Gassman, N.R (2017). Induction of oxidative stress by bisphenol A and its pleiotropic effects. Environ. Mol. Mutagen. 58 (March (2)), 60–71. https://doi.org/10.1002/ em.22072.
Heo, H.J and Lee, C.Y. (2004). Protective effects of quercetin and vitamin C against oxidative stress-induced neurodegeneration. J. Agric. Food Chem. 52 (December (25), 7514–7517. https://doi.org/10.1021/jf049243r.
Inadera, H (2015). Neurological effects of bisphenol A and its analogues. Int. J. Med. Sci. 12 (October (12)), 926–936. https://doi.org/10.7150/ijms.13267.
Ishisaka, A., Ichikawa, S., Sakakibara, H et al. (2011). Accumulation of orally administered quercetin in brain tissue and its antioxidative effects in rats. Free Radic. Biol. Med. 51 (October (7)), 1329–1336. https://doi.org/10.1016/j. freeradbiomed.2011.06.017.
Kabuto, H., Hasuike, S., Minagawa, N et al. (2003). Effects of bisphenol A on the metabolisms of active oxygen species in mouse tissues. Environ. Res. 93 (September (1)), 31–35. https://doi.org/10.1016/s0013-9351(03)00062-8.
Kajta, M and W´ojtowicz, A.K. (2013). Impact of endocrine-disrupting chemicals on neural development and the onset of neurological disorders. Pharmacol. Rep. 65 (6), 1632–1639. https://doi.org/10.1016/s1734-1140(13)71524-x.
Kang, J.H., Asai, D and Katayama, Y (2007). Bisphenol A in the aquatic environment and its endocrine-disruptive effects on aquatic organisms. Crit. Rev. Toxicol. 37 (7), 607–625. https://doi.org/10.1080/10408440701493103.
Kang, J.H., Kondo, F and Katayama, Y (2006). Human exposure to bisphenol A. Toxicology 226 (September (2–3), 79–89. https://doi.org/10.1016/j.tox.2006.06.009.
Kawato, S (2004). Endocrine disrupters as disrupters of brain function: a neurosteroid viewpoint. Environ. Sci. 11 (1), 1–14.
Kelly, K.A., Havrilla, C.M., Brady, T.C et al. (1998). Oxidative stress in toxicology: established mammalian and emerging piscine model systems. Environ. Health Perspect. 106 (July (7)), 375–384. https://doi.org/10.1289/ehp.98106375.
Kuo, Y.C and Tsao, C.W (2017). Neuroprotection against apoptosis of SK-N-MC cells using RMP-7- and lactoferrin-grafted liposomes carrying quercetin. Int. J. Nanomed. 12 (April), 2857–2869. https://doi.org/10.2147/IJN.S132472.
Lorber, M., Schecter, A., Paepke, O et al. (2015). Exposure assessment of adult intake of bisphenol A (BPA) with emphasis on canned food dietary exposures. Environ. Int. 77 (April), 55–62. https://doi.org/10.1016/j. envint.2015.01.008.
Magno, L.D., Fontes, A., Gonçalves, B.M et al. (2015). Pharmacological study of the light/dark preference test in zebrafish (Danio rerio): waterborne administration. Pharmacol. Biochem. Behav. 135 (August), 169–176. https://doi. org/10.1016/j.pbb.2015.05.014.
Mohanty, R., Das, S.K and Patri, M (2017). Modulation of benzo[a]pyrene induced anxiolytic-like behavior by retinoic acid in zebrafish: involvement of oxidative stress and antioxidant defense system. Neurotox. Res. 31 (May (4)), 493–504. https://doi. org/10.1007/s12640-016-9694-5.
Mohanty, R., Das, S.K., Singh, N.R. et al. (2016). Withania somnifera leaf extract ameliorates benzo[a]pyrene-induced behavioral and neuromorphological alterations by improving brain antioxidant status in zebrafish (Danio rerio). Zebrafish 13 (June (3)), 188–196. https://doi.org/10.1089/zeb.2015.1215.
Murata, M and Kang, J.H (2018). Bisphenol A (BPA) and cell signaling pathways. Biotechnol. Adv. 36 (January–February (1)), 311–327. https://doi.org/10.1016/j. biotechadv.2017.12.002.
Nagel, S.C and Bromfield, J.J (2013). Bisphenol A: a model endocrine disrupting chemical with a new potential mechanism of action. Endocrinology 154 (June (6)), 1962–1964. https://doi.org/10.1210/en.2013-1370.
Negri-Cesi, P (2015). Bisphenol A interaction with brain development and functions. Dose 13 (June (2)). https://doi.org/10.1177/1559325815590394, 1559325815590394
Nishikawa, M., Iwano, H., Yanagisawa, R et al. (2010). Placental transfer of conjugated bisphenol A and subsequent reactivation in the rat fetus. Environ. Health Perspect. 118 (September (9)), 1196–1203. https://doi.org/ 10.1289/ehp.0901575.
Pabst, M.J., Habig, W.H and Jakoby, W.B. (1974). Glutathione S-transferase A. A novel kinetic mechanism in which the major reaction pathway depends on substrate concentration. J Biol Chem. 249 (November (22)), 7140–7147.
Rahal, A., Kumar, A., Singh, V et al. (2014). Oxidative stress, prooxidants, and antioxidants: the interplay. Biomed Res. Int. 2014, 761264 https://doi.org/10.1155/2014/761264.
Rennekamp, A.J and Peterson, R.T (2015). 15 years of zebrafish chemical screening. Curr. Opin. Chem. Biol. 24 (February), 58–70. https://doi.org/10.1016/j. cbpa.2014.10.025.
Rochester, J.R. (2013). Bisphenol A and human health: a review of the literature. Reprod. Toxicol. 42 (December), 132–155. https://doi.org/10.1016/j.reprotox.2013.08.008.
Sangai, N.P., Verma, R.J and Trivedi, M.H. (2014). Testing the efficacy of quercetin in mitigating bisphenol A toxicity in liver and kidney of mice. Toxicol. Ind. Health 30 (August (7)), 581–597. https://doi.org/10.1177/0748233712457438.
Sarkar, S., Mukherjee, S., Chattopadhyay, A et al. (2014). Low dose of arsenic trioxide triggers oxidative stress in zebrafish brain: expression of antioxidant genes. Ecotoxicol. Environ. Saf. 107 (September), 1–8. https://doi.org/10.1016/j. ecoenv.2014.05.012.
Serra, E.L., Medalha, C.C and Mattioli, R (1999). Natural preference of zebrafish (Danio rerio) for a dark environment. Braz. J. Med. Biol. Res. 32 (December (12)), 1551–1553. https://doi.org/10.1590/s0100-879×1999001200016.
Winston, G.W (1991). Oxidants and antioxidants in aquatic animals. Comp. Biochem. Physiol. C 100 (1–2), 173–176. https://doi.org/10.1016/0742-8413(91)90148-m.
Wong, Y.M., Li, R., Lee, C.K.F et al. (2017). The measurement of bisphenol A and its analogues, perfluorinated compounds in twenty species of freshwater and marine fishes, a time-trend comparison and human health-based assessment. Mar. Pollut. Bull. 124 (November (2)), 743–752. https://doi.org/ 10.1016/j.marpolbul.2017.05.046.
Xu, D., Hu, M.J., Wang, Y.Q et al. (2019). Antioxidant activities of quercetin and its complexes for medicinal application. Molecules 24 (6). https://doi.org/10.3390/ molecules24061123 pii: E1123.
Yamazaki, E., Yamashita, N., Taniyasu, S et al. (2015). Bisphenol A and other bisphenol analogues including BPS and BPF in surface water samples from Japan, China, Korea and India. Ecotoxicol. Environ. Saf. 122 (December), 565–572. https://doi.org/10.1016/j.ecoenv.2015.09.029.
Zhou, Y., Wang, Z, Xia, M., et al. (2017). Neurotoxicity of low bisphenol A (BPA) exposure for young male mice: implications for children exposed to environmental levels of BPA. Environ. Pollut. 229 (October), 40–48. https://doi.org/10.1016/j.envpol.2017.05.043.
Zimmers, S.M., Browne, E.P., O’Keefe et al. (2014). Determination of free bisphenol A (BPA) concentrations in breast milk of U.S. women using a sensitive LC/MS/MS method. Chemosphere 104 (June), 237–243. https://doi.org/10.1016/j.chemosphere.2013.12.085.gnaling cascades.


