1,3College of Computer, Science and Information Technology, Junagadh, Gujarat
2M. N. Virani Science College, Rajkot, Gujarat
Corresponding author email: dharmesh.microbio@gmail.com
Article Publishing History
Received: 19/04/2020
Accepted After Revision: 29/05/2020
In last several decades the properties of soil are damaged due to modern agricultural practices. Synthetic fertilizers damaged the natural microbial flora of soil which was maintaining the fertility of the soil. In present study we were characterization the plant growth promoting rhizobacteria isolated from the rhizospheric area of wheat (Triticum aestivum L.) of Saurastra region of Gujarat, India. All the isolates were screened for plant growth promoting trait to utilize them for the sustainable agriculture. Total thirty-four organisms were purified from the three different ecological region (Dhandhusar, Gir-gadhada, Gingani) of Saurashtra region. Among the 34 bacterial isolates twenty-eight were able to produce indole-3- acetic acid in tryptophan supplemented medium; twenty were able to solubilize inorganic phosphate and zinc in vitro. PE-1was found to produce high amount of IAA i.e. 46.01 µg/ml, JG-13 solubilizes maximum inorganic phosphate (635 µgml−1) followed by GG-12 (603 µgml−1), JZ-8 gives 25mm zone on ZnO2 medium around colony. Present study indicates the potentiality of PGPR that can be utilize as a biofertilizer for better enhancement of productivity and health of wheat crop.
Rhizobacteria, Wheat, IAA, Phosphate
Sherathia D, Jadeja V, Aghera B, Vasvelia B, Jethava B. Characterization of Potential Plant Growth Promoting Rhizobacteria Excerpted From Wheat, Triticum aestivum Rhizosphere of Saurashtra Region. Biosc.Biotech.Res.Comm. 2020;13(2).
Sherathia D, Jadeja V, Aghera B, Vasvelia B, Jethava B. Characterization of Potential Plant Growth Promoting Rhizobacteria Excerpted From Wheat, Triticum aestivum Rhizosphere of Saurashtra Region. Biosc.Biotech.Res.Comm. 2020;13(2). Available from: https://bit.ly/2xJPJCT
Copyright © Sherathia et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
In Saurashtra region, wheat is another major crop of winter season. Many biotic and abiotic factors affected on the production rate of wheat. The state agriculture department has estimated wheat production in Gujarat in the financial year 2016 to be at about 4.80 million tonnes that is 5 percent of total production in India. The overall production of wheat is set back by some biotic factors (plant pathogens) and abiotic factor (environmental factor). Therefore, there is an urgent need to find alternative strategies that can ensure competitive crop yields, provide environmental safety, and protection while maintain long term ecological balance in agro-ecosystem. Use of microbial inoculants or plant growth promoting rhizobateria (PGPR) for the enhancement of sustainable agricultural production is becoming a more widely accepted practice in intensive agriculture in many parts of the world, (Alori and Babalola 2018, Santos et al 2019).
Plant growth promoting rhizobacteria are free living soil bacteria that aggregate around rood surface (rhizosphere) and enhance the growth and yield of crops when directly applied on seeds (Kumar et al., 2014). The bacteria that provide some beneficial effect to the plants are of two types, those that form a symbiotic relationship with the plant and those that are free living in the soil, but are often found on or near to the roots and inside the roots (Kloepper et al., 1988, Van Peer and Schippers, 1989). Beneficial free-living bacteria are called plant growth promoting rhizobacteria or PGPR (Kloepper et al., 1989). PGPR can affect plant growth in two ways, indirectly or directly. There are many mechanisms by which PGPR exhibit plant growth promotion. PGPR can fix nitrogen and supply it to plants; they synthesize siderophores that can sequester iron from soil and provide it to plant cells; produce phytohormones and solubilize inorganic phosphorus and low molecular mass compounds (Davison, 1988; Lambert and Joos 1989; Glick et al., 1994a, 1994b), ammonification, etc. In our recent study was planned to isolate rhizosperic bacterial of wheat from different climatic location and examine their role in plant growth promotion. These bacteria were screened for their in-vitro potentiality by biochemical test. In our further study we are going to examine their role along with some other microbial population.
MATERIALS AND METHODS
Selection of sampling site and isolation of bacteria: Wheat (Triticum aestivum L.) variety LOK-1 were collected from different region of saurashtra region according to physiological and chemical properties of soil for the isolation of rhizospheric bacteria (Table1). Samples were collected from the different geographical location on of Saurashtra region i.e Dhandhusar (21.552687, 70.357483), Gir-gadhada (20.922836, 70.929627), Gingani (21.882330, 70.072294).
Isolation of PGPR from wheat rhizospheric soil: Rhizospheric bacteria were isolated from 1g soil tightly adhering to the root by serial dilution plating on nutrient agar plates and King’s medium B (King et al., 1954) as described (Somasegaranand Hoben, 1994). The plates were incubated at 30 ± 2ºC for 24hrs than up to 48hrs. Individual colonies were picked and streaked on nutrient agar plate for further purification. A total of one hundred and fifty-four Rhizobacterial isolates were obtained. The isolates were incubated at 30 ± 2ºC during culturing and maintained as glycerol stocks (35% glycerol+ KB broth) at -20ºC.
Morphological and biochemical analyses of wheat PGPR: Colony morphology, size, colour, shape, gum production, and growth pattern were recorded after 24hrs of growth on King’B agar plate at 30 ± 2ºC as described by Somasegaran and Hoben (1994) with some modification. Cell size and motility was observed by light microscopy. Acid/alkali production was tested on nutrient broth containing phenol red as pH indicator. Gram’s reaction was carried out as described by Vincent and Humphrey (1970).
Bioassay for plant growth promotion activity: All isolates collected from wheat rhizosphere were screened for detecting and quantifying the production of indole acetic acid (IAA) like substances. All rhizobacterial isolates were grown in 100 ml Erlenmeyer flasks containing 25 ml of Tryptone yeast extract (TY) broth and incubated on a rotary shaker in the dark at 30 ± 2ºC for 4 days (Sarwar and Kremer (1995). Than 25 ml of culture broth were centrifuged at 2000 rpm for 20 mins to remove cell debris. 1ml of clear supernatant was transfer in another tube to determine concentration of IAA and add 1 ml of Salkowsky reagent (1 ml of 0.5 M FeCl3 in 50 ml of 3.5% HClO4) to developed colour. The reaction mixture was incubated in the dark for 1h. Pink colour developed which conformed which IAA production. Amount of IAA was quantified by taking supernatant in 1 ml of the reagent, the reaction mixture was incubated in the dark for 60 min for colour development.
Absorbance was taken in visible spectrophotometer at 535 nm. The amount of IAA produced was measured from the standard curve. Solubilisation of insoluble phosphate by the rhizobacterial isolates was tested in Pikovskaya’s medium (Pikovskaya, 1948), containing tri-calcium phosphate. Spot inoculation was done with the rhizobacterial isolates and plates were incubated at 32°C for 4-7 days. Observation was taken for clearing or solubilization zone around the colonies. The solubilization of tri-calcium phosphate was quantified in Pikovskaya’s broth (Pikovskaya, 1948), following the protocol described by Dey et al. (2004). The result of solubilisation in measured after 6 days of incubation.
RESULTS AND DISCUSSION
Isolation and morphological analysis of wheat rhizospheric bacteria: Bacteria were isolated form the rhizosphere of wheat (Triticum aestivum L.) during winter season from the three different location of Saurashtra region (Table1). Total 65 organisms were isolated after observing colony morphology. Finally, after observing colony morphology different 34 colonies were selected for further study. The bacteria showed mostly are non-pigmented and others give pigments like bluish, yellow, and greyish with variable sizes, margins and elevation on KB’s agar plates. Shape of cells were short rods, long rods and cocci observed under light microscope. Twenty organisms gives gram’s positive reaction and sixteen gram’s negative reaction with KOH solution and grams staining.In microscopic observation using several techniques like gram staining, negative staining and hanging drop method of different isolates are illustrated in table 2. Total 24 isolates were motile, six were cocci shaped and others were short rods or long rods. Bacterial with different colony morphology were selected and maintained on nutrient agar slants and 60% glycerol at -40°C.
Table 1. Geographical location of rhizosperic isolates
| Sampling site | Geographical location | Isolates ID |
| Dhandhusar, Dist. Junagadh, Gujarat, India | 21.552687, 70.357483 | GG-1, GG-2, GG-3, GG-4, GG-5
GG-6, GG-7, GG-8, GG-9, GG-10 GG-11, GG-12 |
| Gir-gadhada, Dist. Junagadh, Gujarat, India | 20.922836, 70.929627 | JZ-1, JZ-2, JZ-3, JZ-4, JZ-5, JZ-6
JZ-7, JZ-8 |
| Gingani, Dist. Junagadh, Gujarat, India | 21.882330, 70.072294 | JG-1, JG-2, JG-3, JG-4, JG-5, JG-6
JG-7, JG-8, JG-9, JG-10, JG-11, JG-12, JG-13, JG-14 |
Colony morphology and cell morphology of wheat rhizospheric bacteria: All organisms show different colony morphology viz. brown, green, yellowish pigmentation, round, oval shape, small to large size colony and viscous, moist, dry like consistency were observed in different isolates of wheat rhizosphere. Regarding cell shape and gram-staining, 20 isolates were gram-positive and 14 were gram-negative. Long rod, short rod and cocci shaped cell morphology were observed under microscopic observation (Table 2).
Table 2. Colony morphology of wheat rhizosperic isolates.
| Sr. No. | Isolates ID | Gram’s Nature | Cell Shape | Motility |
| 1 | GG-1 | Gram-Negative | Cocci | Motile |
| 2 | GG-2 | Gram-Positive | Long Rod | Non-motile |
| 3 | GG-3 | Gram-Positive | Long Rod | Non-motile |
| 4 | GG-4 | Gram-Positive | Rod | Motile |
| 5 | GG-5 | Gram positive | Rod | Motile |
| 6 | GG-6 | Gram positive | Rod | Motile |
| 7 | GG-7 | Gram positive | Short rod | Motile |
| 8 | GG-8 | Gram positive | Rod | Motile |
| 9 | GG-9 | Gram-Positive | Rod | Motile |
| 10 | GG-10 | Gram-Positive | Rod | Non-motile |
| 11 | GG-11 | Gram-Positive | Rod | Non-motile |
| 12 | GG-12 | Gram-Negative | Short Rod | Motile |
| 13 | JZ-1 | Gram positive | Rod | Non motile |
| 14 | JZ-2 | Gram positive | Rod | Motile |
| 15 | JZ-3 | Gram positive | Long rod | Non motile |
| 16 | JZ-4 | Gram positive | Rod | Motile |
| 17 | JZ-5 | Gram positive | Rod | Motile |
| 18 | JZ-6 | Gram positive | Rod | Motile |
| 19 | JZ-7 | Gram positive | Short Rod | Non motile |
| 20 | JZ-8 | Gram negative | Rod | Motile |
| 21 | JG-1 | Gram-Negative | Short Rod | Motile |
| 22 | JG-2 | Gram-Negative | Short Rod | Non-motile |
| 23 | JG-3 | Gram-Positive | Long Rod | Motile |
| 24 | JG-4 | Gram-Negative | Short Rod | Motile |
| 25 | JG-5 | Gram-Negative | Short Rod | Non-motile |
| 26 | JG-6 | Gram-Negative | Cocci | Motile |
| 27 | JG-7 | Gram-Negative | Short Rod | Motile |
| 28 | JG-8 | Gram-Negative | Short Rod | Motile |
| 29 | JG-9 | Gram-Negative | Long Rod | Motile |
| 30 | JG-10 | Gram-Negative | Cocci | Motile |
| 31 | JG-11 | Gram-Positive | Filamentous | Non-motile |
| 32 | JG-12 | Gram-Positive | Cocci | Motile |
| 33 | JG-13 | Gram-Negative | Cocci | Motile |
| 34 | JG-14 | Gram-Negative | Cocci | Motile |
Carbohydrate utilization efficiency of Wheat rhizospheric bacteria:Carbohydrate utilization like Glucose, Sucrose, Lactose and Mannitol were tested on all selected PGPR. It was observed that out of 34 isolates 22 having capacity to utilize glucose. In carbohydrate utilization test glucose, lactose, sucrose, mannitol sugars were supplemented in media and noticed glucose was utilized by the 22 isolates, Lactose was utilized by the 23 isolates and Sucrose was utilized by the 25 isolates and Mannitol was utilized by the 22 isolates. Not all glucose utilizer was able to produced acid through carbohydrate metabolism.
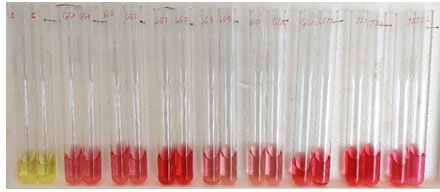
Figure 1: IAA production by PGPR
IAA production of wheat rhizospheric bacteria
The ability of PGPR to produce IAA found in different species had reported earlier (Mansour et al., 1994; Zahir et al., 2000). The various pathways, enzymes complexes and genes involved for the biosynthesis of IAA (Patten and Glick, 1996). The production of IAA by bacteria isolated from rhizosphere of different crops, i.e., peanut, wheat, and rice had already been reported in number of studies (Dey et al., 2004; A. Khalid et al. 2004, Prakash Nathan et al. 2011).
Figure 2: Zinc Solubilization by PGPR
Out of thirty-six isolates twenty-eight were able to synthesis the IAA from the tryptophan amino acids. IAA produced by all isolates ranged in between 3.15 µg/ml to 46.01µg/ml. Among all isolates, JG-7 was found to produce high amount of IAA i.e. 46.01µg/ml. some of the isolates were not able to produced IAA were GG-3, GG-4, GG-5, GG-6, GG-9, GG-11, JZ-4, JZ-5, JG-10. 74% of total population were able to produce plant growth hormone, indole acetic acid. In vitro plant growth promotion traits of the rhizobacteria are described in Table 2.
Phosphate and Zinc solubilisation by wheat rhizospheric bacteria: Quantitative estimation of P-solubilizing activity was done in Pikovskaya’s medium (Sherathia et al. 2016). Phosphorus is the second most important nutrient, next to nitrogen (N) required for growth of plants. A greater portion of phosphorus in soil is in the form of insoluble phosphates and cannot be used directly by the plants (Pradhan et al. 2006). In the above study, isolates were found to give clear zone on Pikovskaya agar containing insoluble mineral phosphate such as tri-calcium phosphate (Table 3). More or less this isolates were able to utilize zinc also. Several researchers proved that the phosphate solubilizers are more around the rhizosphere in comparison to bulk soil.
Figure 3: Phosphate Solubilization
Twenty bacteria were able to give form clear zone on Pikovskaya agar plates after 7 days of incubation. All twenty isolates were quantified for phosphate solubilisation in tri-calcium phosphate liquid media. From the all isolates JG-13 solubilize maximum inorganic phosphate JG-13 (635 µgml−1) followed by GG-2 (603 µgml−1). Another were also able to solubilized good amount of inorganic phosphate. Zinc solubilisation ability of the bacterial strains was evaluated by determining the zone of solubilisation of zinc (ZnO2). About 31% of total isolates were show more than 10mm zone around colony and 40% were not solubilize zinc. GG-12 shown maximum zinc solubilisation (29mm) Medium, JZ-8 gives 25mm zone on ZnO2 medium. All 34 isolates solubilize zinc on ZnO2 supplemented medium (Table 3)
Table 3. Plant growth promotional traits of rhizosperic isolates
| Phosphate Solubilisation | Zinc Solubilisation | IAA production | |||
| Sr. No. | Isolates ID | Zone of clearance(mm) | Concentration
(µg/ml) |
Zone of clearance(mm) | Concentration
(µg/ml) |
| 1 | GG-1 | 11 | 268 | 6 | 8.02 |
| 2 | GG-2 | 17 | 483 | 15 | 7.60 |
| 3 | GG-3 | 10 | 244 | 5 | 0.00 |
| 4 | GG-4 | 14 | 362 | 10 | 0.00 |
| 5 | GG-5 | 0 | 0 | 0 | 0.00 |
| 6 | GG-6 | 3 | 42 | 10 | 0.00 |
| 7 | GG-7 | 20 | 552 | 16 | 28.50 |
| 8 | GG-8 | 0 | 0 | 0 | 7.60 |
| 9 | GG-9 | 7 | 122 | 0 | 0.00 |
| 10 | GG-10 | 6 | 141 | 3 | 5.43 |
| 11 | GG-11 | 0 | 0 | 2 | 0.00 |
| 12 | GG-12 | 26 | 603 | 29 | 12.44 |
| 13 | JZ-1 | 0 | 0 | 0 | 34.88 |
| 14 | JZ-2 | 6 | 89 | 20 | 12.71 |
| 15 | JZ-3 | 0 | 0 | 10 | 11.76 |
| 16 | JZ-4 | 0 | 0 | 0 | 0.00 |
| 17 | JZ-5 | 7 | 136 | 0 | 0.00 |
| 18 | JZ-6 | 0 | 0 | 0 | 17.97 |
| 19 | JZ-7 | 10 | 236 | 10 | 13.45 |
| 20 | JZ-8 | 19 | 489 | 25 | 12.50 |
| 21 | JG-1 | 10 | 263 | 6 | 40.74 |
| 22 | JG-2 | 0 | 0 | 0 | 17.66 |
| 23 | JG-3 | 0 | 0 | 0 | 15.22 |
| 24 | JG-4 | 14 | 342 | 7 | 36.66 |
| 25 | JG-5 | 0 | 0 | 0 | 3.49 |
| 26 | JG-6 | 0 | 0 | 13 | 11.17 |
| 27 | JG-7 | 20 | 485 | 9 | 46.13 |
| 28 | JG-8 | 11 | 242 | 0 | 29.31 |
| 29 | JG-9 | 0 | 0 | 3 | 3.11 |
| 30 | JG-10 | 0 | 0 | 0 | 0 |
| 31 | JG-11 | 5 | 103 | 0 | 8.33 |
| 32 | JG-12 | 0 | 0 | 0 | 19.57 |
| 33 | JG-13 | 32 | 635 | 0 | 26.45 |
| 34 | JG-14 | 15 | 326 | 0 | 5.98 |
REFERENCES
Alori, E.T and O.O. Babalola (2018) Microbial Inoculants for Improving Crop Quality and Human Health in Africa Front. Microbiol., 19 September 2018 | https://doi.org/10.3389/fmicb.2018.02213
Pradhan, N. and Sukla, L. B. (2006) Solubilization of inorganic phosphates by fungi isolated from agriculture soil, African Journal of Biotechnology, 5, pp. 850-854.
Mansour, F. A., Ildesuguy, H. S. and Hamedo, H. A. (1994) Studies on plant growth regulators and enzyme production by some bacteria, Journal of Qatar University Science, 14, pp. 81-288.
Zahir, A., Abbas, S. A., Khalid, M., and Arshad, M. (2000) Structure dependent microbially derived plant hormones by improving growth of maize seedlings, Pakistan Journal of Biological Science 3, pp. 289–291
Patten, C.L. and Glick, B.R. (2002) Role of Pseudomonas putida indole-acetic acid in development of the host plant root system. Applied Environmental Microbiology, 68 pp. 3795-3801.
Nathan, P., Rathinam, X., Kasi, M., Rahman, Z. A., & Subramaniam, S. (2011) A pilot study on the isolation and biochemical characterization of Pseudomonas from chemical intensive rice ecosystem, African Journal of Biotechnology, 10(59), pp. 12653–12656.
Somasegaran, P., and Hoben, H. J. (1994) Handbook for Rhizobia, Methods in Legume–Rhizobium Technology. Heidelberg, NY: Springer. doi: 10.1007/978-1- 4613-8375-8.
King, E. O., Ward, M. K. and Raney, D. E. (1954) Two simple media for demonstration of pyocyanin and fluorescein, Journal of Laboratory and Clinical Medicine, 44, pp. 301-307.
Vincent, J. M., and Humphrey, B. (1970) Taxonomically significant group antigens in Rhizobium. J. Gen. Microbiol. 63, pp. 379–382. doi: 10.1099/00221287-63-3-379.
Dey, R., Pal, K.K., Bhatt, D.M. and Chauhan, S.M. (2004). Growth promotion and yield enhancement of peanut (Arachis hypogaea L.) by application of plant growth promoting rhizobacteria. Microbiol. Res., 159, pp. 371-394.
Kumar, A., Maurya, B. R. and Raghuwanshi, R. (2014) Isolation and characterization of PGPR and their effect on growth, yield and nutrient content in wheat (Triticum aestivum L.). Biocatalysis and Agricultural Biotechnology, 3, pp. 121–128.
Kloepper, J. W., Lifshitz, R. and Schroth, M. N. (1988). Pseudomonas inoculants to benefit plant production. ISI Atlas of Science: Animal and Plant Sciences pp. 60-64.
Lambert, B. and Joos, H. (1989). Fundamental aspects of rhizobacterial plant growth promotion research. Trands in Biotechnology, 7, pp. 215-219.
Van Peer, R. and Schippers, B. (1989). Plant growth responses to bacterization with selected Pseudomonas spp. strains and rhizosphere microbial development in hydroponic cultures, Canadian Journal of Microbiology, 35, pp. 456-463.
Kloepper, J. W., Lifshitz, R. and Zablotowicz, R. M. (1989). Free-living bacterial inocula for enhancing crop productivity, Trends in Biotechnology, 7, pp. 39-44.
Davison, J. (1988). Plant beneficial bacteria, Bio/ Technology, 6, pp. 282-286.
Glick, B. R., Jacobson, C. B., Schwarze, M. M. K. and Pasternak, J. J. (1994a) Does the enzyme 1-aminocyclopropane-1-carboxylate deaminase play a role in plant growth-promotion by Pseudomonas putida GR12-2? In Improving plant productivity with rhizosphere bacteria. M. H. Ryder, P. M. Stephens, and G. D. Bowen (Eds.). Common Wealth Scientific and Industrial Research Organisation, Adelaide, Australia pp. 150-152.
Glick, B. R., Jacobson, C. B., Schwarze, M. M. K. and Pasternak, J. J. (1994b) 1-Aminocyclopropane-1-carboxylic acid deaminase mutants of the plant growth promoting rhizobacterium Pseudomonas putida GR12-2 do not stimulate canola root elongation. Canadian Journal of Microbiology, 40, pp. 911-915.
Santos MS, Nogueira MA and M Hungria (2019) Microbial inoculants: reviewing the past, discussing the present and previewing an outstanding future for the use of beneficial bacteria in agriculture AMB Express Volume 9, Article number: 205
Sherathia, D., Dey, R., Thomas, M., Dalsania, T., Savsani, K., Pal, K. K. (2016) Biochemical and molecular characterization of DAPG-producing plant growth-promoting rhizobacteria (PGPR) of groundnut (Arachis hypogaea L.). Legume Research-An International Journal, 39, pp. 614-622.