Abstract
Background: Cancer-related and non-cancer chronic pain embodies the most frequent challenge in clinical practice. Management of chronic pain and breakthrough pain (BTP) requires adjustments to the analgesic regimen to achieve adequate pain control.
Objective: To examine the time to achieve pain relief in patients who experienced intense episodic pain breakouts despite baseline therapy with analgesics.
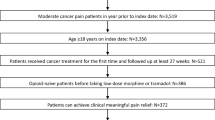
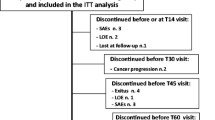
Methods: This study was based on a 14-day observation period. Patients with either cancer-related or non-cancer pain who experienced >2 intensive episodic pain breakouts per day were prescribed immediate-release (IR) morphine sulphate (10 mg to 20 mg as needed) every 4 hours (around-the-clock) and allowed one rescue dose of IR morphine (equal to one additional administration of the dosage taken at fixed times) for any episodic pain breakouts. Patients recorded time of administration and time taken to achieve partial or total relief of episodic pain breakout in daily diaries; in one study centre the diary was managed with the help of specialized medical attendants. Pain intensity and general wellbeing were assessed by a Numerical Rating Scale (NRS) and Karnofsky Performance Scale, respectively. Adverse events and sleep patterns were also recorded.
Results: Of 85 patients (mean age 62.67 ± 14.3 years) enrolled, 14 experienced pain from non-cancer degenerative diseases, and 71 had cancer-related pain. Following stabilization of background pain, the intensity of daily pain improved; NRS decreased from baseline to day 14 for cancer (from 5.63 to 1.98) and non-cancer (from 8.00 to 1.00) groups (both p < 0.0001). Patients’ general wellbeing increased concomitantly. Around-the-clock therapy resulted in an immediate decrease in the number of intense episodic pain breakouts per day, with 11.8% of patients achieving total pain relief within 24 hours. The mean number of intense episodic pain breakouts per day decreased steadily in the cancer group, reaching significance at day 14 (p < 0.001 vs baseline). Moreover, the time to achieve partial and total pain relief of intense episodic pain breakouts improved significantly. Adverse events and sleep patterns improved over the 14-day observation period.
Conclusions: Stabilization of background cancer-related or non-cancer pain with around-the-clock IR morphine therapy resulted in fewer intense episodic pain breakouts, which were more quickly managed with rescue-dose IR morphine, suggesting that ‘end-dose’ pain should not be classified as BTP.






Similar content being viewed by others
References
Zeppetella G. Impact and management of breakthrough pain in cancer. Curr Opin Support Palliat Care 2009 Mar; 3(1): 1–6
Hanks GW, Conno F, Cherny N, et al. Morphine and alternative opioids in cancer pain: the EAPC recommendations. Br J Cancer 2001 Mar 2; 84(5): 587–93
Zeppetella G, Ribeiro MD. Opioids for the management of breakthrough (episodic) pain in cancer patients. Cochrane Database Syst Rev 2006; (1): CD004311
Portenoy RK, Hagen NA. Breakthrough pain: definition, prevalence and characteristics. Pain 1990 Jun; 41(3): 273–81
Mercadante S, Radbruch L, Caraceni A, et al. Episodic (breakthrough) pain: consensus conference of an expert working group of the European Association for Palliative Care. Cancer 2002 Feb 1; 94(3): 832–9
Bennett D, Burton AW, Fishman S, et al. Consensus panel recommendations for the assessment and management of breakthrough pain. Part 2: management. P & T 2005; 30(6): 354–61
Davies AN, Dickman A, Reid C, et al. The management of cancer-related breakthrough pain: recommendations of a task group of the Science Committee of the Association for Palliative Medicine of Great Britain and Ireland. Eur J Pain 2009 Apr; 13(4): 331–8
Casuccio A, Mercadante S, Fulfaro F. Treatment strategies for cancer patients with breakthrough pain. Expert Opin Pharmacother 2009 Apr; 10(6): 947–53
DeConno F, Ripamonti C, Fagnoni E, et al. The MERITO Study: a multicentre trial of the analgesic effect and toler-ability of normal-release oral morphine during ‘titration phase’ in patients with cancer pain. Palliat Med 2008 Apr; 22(3): 214–21
Ripamonti CI, Campa T, Fagnoni E, et al. Normal-release oral morphine starting dose in cancer patients with pain. Clin J Pain 2009 Jun; 25(5): 386–90
Gatti A, Reale C, Occhioni R, et al. Standard therapy with opioids in chronic pain management: ORTIBER study. Clin Drug Investig 2009; 29Suppl. 1: 17–23
Fortner BV, Okon TA, Portenoy RK. A survey of pain-related hospitalizations, emergency department visits, and physician office visits reported by cancer patients with and without history of breakthrough pain. J Pain 2002 Feb; 3(1): 38–44
Geppetti P, Benemei S. Pain treatment with opioids: achieving the minimal effective and the minimal interacting dose. Clin Drug Investig 2009; 29Suppl. 1: 3–16
McCarberg BH. The treatment of breakthrough pain. Pain Med 2007 Jan–Feb; 8Suppl. 1: S8–13
Zeppetella G. Opioids for cancer breakthrough pain: a pilot study reporting patient assessment of time to meaningful pain relief. J Pain Symptom Manage 2008 May; 35(5): 563–7
Jumbelic MI. Deaths with transdermal fentanyl patches. Am J Forensic Med Pathol 2010 Mar; 31(1): 18–21
Acknowledgements
The authors have no conflicts of interest relating to the contents of this article; no external funding was received. English-language and editorial assistance for the preparation of this article was provided by Melanie Gatt of inScience Communications, a Wolters Kluwer business, and was funded by Molteni Farmaceutici, Inc. We thank Ennio Sarli (Centro Consulenze, Florence, Italy) for data management and Cristina Camisola (Intensive Care Antalgic Therapy Hospice Unit, San Camillo de’Lellis Hospital, Rieti, Italy) for coordinating the nurses who participated in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Presti, C.L., Roscetti, A., Muriess, D. et al. Time to Pain Relief After Immediate-Release Morphine in Episodic Pain. Clin. Drug Investig. 30 (Suppl 2), 49–55 (2010). https://doi.org/10.2165/1158412-S0-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/1158412-S0-000000000-00000