Abstract
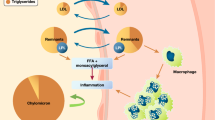
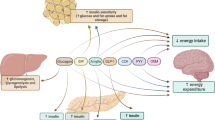
Preventing morbidity and mortality from diabetes mellitus is of paramount importance as the incidence of this disease is increasing across the world. While microvascular complications of diabetes such as nephropathy, retinopathy, and neuropathy are reduced with intensive glycemic control, treatment of hyperglycemia has not been consistently shown to have effects on the macrovascular complications of diabetes such as coronary artery, cerebrovascular, and peripheral vascular disease. Preventive efforts have accordingly shifted toward the modification of other cardiovascular risk factors in diabetic patients. Agonism of the peroxisome proliferator-activated receptors (PPARs) has long been an attractive target for antidiabetic therapy due to the role of PPARs in glycemic control and lipid metabolism. PPAR-γ agonists such as rosiglitazone and pioglitazone are used in clinical practice for the treatment of diabetes, and there is some evidence that pioglitazone may have positive effects on cardiovascular complications by virtue of its favorable effects on lipid profiles. However, they have not been shown to reduce macrovascular events. PPAR-α agonism is the mechanism of action in the fibrate class of medications; these agents have been shown to increase high-density lipoprotein cholesterol (HDL-C) levels, reduce triglyceride levels, and improve cardiovascular outcomes. Given the prevalence of lipid abnormalities in patients with diabetes, dual PPAR-α/γ agonists (glitazars) could potentially benefit patients with diabetes. A phase II trial examining a novel dual PPAR agonist, aleglitazar, showed that therapy with this agent reduced hyperglycemia and favorably modified levels of HDL-C and triglycerides with an acceptable safety profile. Aleglitazar is currently being studied in large-scale clinical trials to assess whether it will reduce the risk of major cardiovascular endpoints (death, myocardial infarction, or stroke) among patients with diabetes and coronary artery disease. If ongoing studies confirm the theoretical benefit and safety of dual PPAR-α/γ agonism, aleglitazar may become the first therapy demonstrated to reduce macrovascular complications in patients with diabetes.





Similar content being viewed by others
References
Fauci AS. Harrison’s principles of internal medicine. 17th ed. New York: McGraw-Hill Medical; 2008.
King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care 1998; 21(9): 1414–31.
Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA 2010; 20; 303(3): 235–41.
The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes 1995; 44(8): 968–83.
Kahn R, Robertson RM, Smith R, et al. The impact of prevention on reducing the burden of cardiovascular disease. Circulation 2008; 118(5): 576–85.
Fox CS, Evans JC, Larson MG, et al. Temporal trends in coronary heart disease mortality and sudden cardiac death from 1950 to 1999: the Framingham Heart Study. Circulation 2004; 110(5): 522–7.
Fox CS, Coady S, Sorlie PD, et al. Increasing cardiovascular disease burden due to diabetes mellitus: the Framingham Heart Study. Circulation 2007; 115(12): 1544–50.
The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329(14): 977–86.
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352(9131): 837–53.
Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358(24): 2545–59.
Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010; 362(17): 1563–74.
Cushman WC, Evans GW, Byington RP, et al. Effects of intensive bloodpressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362(17): 1575–85.
Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358(24): 2560–72.
Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360(2): 129–39.
Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359(15): 1577–89.
Kaul S, Bolger AF, Herrington D, et al. Thiazolidinedione drugs and cardiovascular risks: a science advisory from the American Heart Association and American College Of Cardiology Foundation. J Am Coll Cardiol 2010; 55(17): 1885–94.
Turner RC, Millns H, Neil HA, et al. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23). BMJ 1998; 316(7134): 823–8.
Mazzone T, Chait A, Plutzky J. Cardiovascular disease risk in type 2 diabetes mellitus: insights from mechanistic studies. Lancet 2008; 371(9626): 1800–9.
Costa J, Borges M, David C, et al. Efficacy of lipid lowering drug treatment for diabetic and non-diabetic patients: meta-analysis of randomised controlled trials. BMJ 2006; 332(7550): 1115–24.
Adler AI, Stratton IM, Neil HA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ 2000; 321(7258): 412–9.
Ho JS, Cannaday JJ, Barlow CE, et al. Relation of the number of metabolic syndrome risk factors with all-cause and cardiovascular mortality. Am J Cardiol 2008; 102(6): 689–92.
Gaede P, Vedel P, Parving HH, et al. Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno type 2 randomised study. Lancet 1999; 353(9153): 617–22.
Nathan DM. Clinical practice. Initial management of glycemia in type 2 diabetes mellitus. N Engl J Med 2002; 347(17): 1342–9.
Vamecq J, Latruffe N. Medical significance of peroxisome proliferatoractivated receptors. Lancet 1999; 354(9173): 141–8.
Chang F, Jaber LA, Berlie HD, et al. Evolution of peroxisome proliferatoractivated receptor agonists. Ann Pharmacother 2007; 41(6): 973–83.
US FDA. Center for drug evaluation and research application number: 021071. 1999 [online]. Available from URL: http://www.fda.gov [Accessed 2010 Jul 1].
Chappuis B, Braun M, Stettler C, et al. Differential effect of pioglitazone (PGZ) and rosiglitazone (RGZ) on postprandial glucose and lipid metabolism in patients with type 2 diabetes mellitus: a prospective, randomized crossover study. Diabetes Metab Res Rev 2007; 23(5): 392–9.
Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007; 356(24): 2457–71.
Lincoff AM, Wolski K, Nicholls SJ, et al. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA 2007; 298(10): 1180–8.
Juurlink DN, Gomes T, Lipscombe LL, et al. Adverse cardiovascular events during treatment with pioglitazone and rosiglitazone: population based cohort study. BMJ 2009; 339: b2942.
Home PD, Pocock SJ, Beck-Nielsen H, et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet 2009; 373(9681): 2125–35.
Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macrovascular Events): a randomised controlled trial. Lancet 2005; 366(9493): 1279–89.
Krey G, Braissant O, L’Horset F, et al. Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Mol Endocrinol 1997; 11(6): 779–91.
Steiner G. Fibrates and coronary risk reduction. Atherosclerosis 2005; 182(2): 199–207.
Frick MH, Elo O, Haapa K, et al. Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med 1987; 317(20): 1237–45.
Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med 1999; 341(6): 410–8.
Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet 2005; 366(9500): 1849–61.
Henry RR, Lincoff AM, Mudaliar S, et al. Effect of the dual peroxisome proliferator-activated receptor-alpha/gamma agonist aleglitazar on risk of cardiovascular disease in patients with type 2 diabetes (SYNCHRONY): a phase II, randomised, dose-ranging study. Lancet 2009; 374(9684): 126–35.
Bays H, McElhattan J, Bryzinski BS. A double-blind, randomised trial of tesaglitazar versus pioglitazone in patients with type 2 diabetes mellitus. Diab Vasc Dis Res 2007; 4(3): 181–93.
Saad MF, Greco S, Osei K, et al. Ragaglitazar improves glycemic control and lipid profile in type 2 diabetic subjects: a 12-week, double-blind, placebocontrolled dose-ranging study with an open pioglitazone arm. Diabetes Care 2004; 27(6): 1324–9.
Long GG, Reynolds VL, Lopez-Martinez A, et al. Urothelial carcinogenesis in the urinary bladder of rats treated with naveglitazar, a γ-dominant PPAR α/γ agonist: lack of evidence for urolithiasis as an inciting event. Toxicol Pathol 2008; 36: 218–31.
Buse JB, Rubin CJ, Frederich R, et al. Muraglitazar, a dual (alpha/gamma) PPAR activator: a randomized, double-blind, placebo-controlled, 24-week monotherapy trial in adult patients with type 2 diabetes. Clin Ther 2005; 27(8): 1181–95.
Nissen SE, Wolski K, Topol EJ. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA 2005; 294(20): 2581–6.
Sanwald-Ducray P, Liogier D’ardhuy X, Jamois C, et al. Pharmacokinetics, pharmacodynamics, and tolerability of aleglitazar in patients with type 2 diabetes: results from a randomized, placebo-controlled clinical study. Clin Pharmacol Ther. Epub 2010.
Acknowledgments
No funding was received for the preparation of this article. A. Michael Lincoff has received research support from Takeda and Roche. M. Cavender has no conflicts of interest that are directly relevant to the content of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cavender, M.A., Lincoff, A.M. Therapeutic Potential of Aleglitazar, a New Dual PPAR-α/γ Agonist. Am J Cardiovasc Drugs 10, 209–216 (2010). https://doi.org/10.2165/11539500-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11539500-000000000-00000