Abstract
Panitumumab (Vectibix®) is a recombinant, fully human, IgG2 anti-epidermal growth factor receptor (EGFR) monoclonal antibody. This spotlight reviews the clinical efficacy of intravenous panitumumab in combination with chemotherapy in the first- and second-line treatment of metastatic colorectal cancer and as monotherapy in chemotherapy-refractory metastatic colorectal cancer, as well as summarizing its pharmacologic properties and tolerability. Panitumumab is indicated for use in patients with wild-type rather than mutant KRAS tumors.
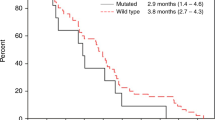
The efficacy of intravenous panitumumab 6 mg/kg administered every 2 weeks was examined in randomized, open-label, multicenter, phase III trials in patients with metastatic colorectal cancer. When administered as first- or second-line treatment in combination with chemotherapy, panitumumab plus chemotherapy prolonged progression-free survival to a significantly greater extent than chemotherapy alone in patients with wild-type KRAS tumors; no significant between-group difference in overall survival was seen in the second-line treatment trial. In patients with mutant KRAS tumors, progression-free survival was significantly shorter with panitumumab plus oxaliplatin-based chemotherapy (FOLFOX4) than with FOLFOX4 alone in the first-line treatment trial, with no significant difference between patients receiving panitumumab plus irinotecan-based chemotherapy (FOLFIRI) and those receiving FOLFIRI alone in the second-line treatment trial. In chemotherapy-refractory patients with metastatic colorectal cancer, panitumumab monotherapy plus best supportive care prolonged progression-free survival to a significantly greater extent than best supportive care alone in both the overall population and in patients with wild-type KRAS tumors, but not in those with mutant KRAS tumors. Intravenous panitumumab has an acceptable tolerability profile when administered as monotherapy or in combination with chemotherapy. It is associated with the skin-related toxicities characteristic of EGFR inhibitors and appears to have a low risk of immunogenicity. In conclusion, in patients with wild-type KRAS tumors, panitumumab is a useful option in combination with chemotherapy for the first- and second-line treatment of metastatic colorectal cancer or as monotherapy for the treatment of chemotherapy-refractory metastatic colorectal cancer.
Similar content being viewed by others
References
Keating GM. Panitumumab: a review of its use in metastatic colorectal cancer. Drugs 2010; 70(8): 1059–78
Foon KA, Yang X-D, Weiner LM, et al. Preclinical and clinical evaluations of ABX-EGF, a fully human anti-epidermal growth factor receptor antibody. Int J Radiat Oncol Biol Phys 2004 Mar 1; 58(3): 984–90
Amgen Inc. Vectibix® (panitumumab) solution for intravenous infusion: US prescribing information [online]. Available from URL: http://www.access-data.fda.gov/drugsatfda_docs/label/2009/125147s080lbl.pdf [Accessed 2010 Jan 14]
Foltz IN, King CT, Liang M, et al. Panitumumab induces internalization of the epidermal growth factor receptor (EGFr) [abstract no. B43]. 17th AACR-NCI EORTC International Conference on Molecular Targets and Cancer Therapeutics; 2005 Nov 14-18; Philadelphia (PA)
Giannopoulou E, Antonacopoulou A, Matsouka P, et al. Autophagy: novel action of panitumumab in colon cancer. Anticancer Res 2009 Dec; 29(12): 5077–82
Yang X-D, Jia X-C, Corvalan JRF, et al. Eradication of established tumors by a fully human monoclonal antibody to the epidermal growth factor receptor without concomitant chemotherapy. Cancer Res 1999 Mar 15; 59(6): 1236–43
Lynch DH, Yang X-D. Therapeutic potential of ABX-EGF: a fully human anti-epidermal growth factor receptor monoclonal antibody for cancer treatment. Semin Oncol 2002 Feb; 29(1 Suppl. 4): 47–50
Freeman D, McDorman K, Bush T, et al. Mono- and combination-therapeutic activity of panitumumab (ABX-EGF) on human A431 epidermoid and HT-29 colon carcinoma xenografts: correlation with pharmacodynamic parameters [abstract no. 313]. 16th EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics; 2004 Sep 28–Oct 1; Geneva
Ma P, Yang B-B, Wang Y-M, et al. Population pharmacokinetic analysis of panitumumab in patients with advanced solid tumors. J Clin Pharmacol 2009 Oct; 49(10): 1142–56
Siena S, Cassidy J, Tabernero J, et al. Randomized phase III study of panitumumab (pmab) with FOLFOX4 compared to FOLFOX4 alone as first-line treatment (tx) for metastatic colorectal cancer (mCRC): PRIME trial [abstract no. 283]. 2010 Gastrointestinal Cancers Symposium; 2010 Jan 22–24; Orlando (FL)
Hecht JR, Mitchell E, Chidiac T, et al. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol 2009 Feb 10; 27(5): 672–80
Peeters M, Price TJ, Hotko YS, et al. Randomized phase III study of panitumumab (pmab) with FOLFIRI versus FOLFIRI alone as second-line treatment (tx) in patients (pts) with metastatic colorectal cancer (mCRC): patient-reported outcomes (PRO) [abstract no. 282]. 2010 Gastrointestinal Cancers Symposium; 2010 Jan 22–24; Orlando (FL)
Peeters M, Price T, Hotko Y, et al. Randomized phase 3 study of panitumumab (pmab) with FOLFIRI vs FOLFIRI alone as second-line treatment (tx) in patients with metastatic colorectal cancer (mCRC) [abstractno. 14LBA]. 15th European Cancer Conference and the 34th Congress of the European Society for Medical Oncology; 2009 Sep 20–24; Berlin
Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 2007 May 1; 25(13): 1658–64
Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 2008 Apr 1; 26(10): 1626–34
Hecht JR, Mitchell E, Neubauer MA, et al. Lack of correlation between epidermal growth factor receptor status and response to panitumumab mono-therapy in metastatic colorectal cancer. Clin Cancer Res 2010 Apr 1; 16(7): 2205–13
Muro K, Yoshino T, Doi T, et al. A phase 2 clinical trial of panitumumab monotherapy in Japanese patients with metastatic colorectal cancer. Jpn J Clin Oncol 2009 May; 39(5): 321–6
Hecht JR, Patnaik A, Berlin J, et al. Panitumumab monotherapy in patients with previously treated metastatic colorectal cancer. Cancer 2007 Sep 1; 110(5): 980–8
Amado RG, Wolf M, Freeman D, et al. Association of KRAS mutational status and efficacy of panitumumab monotherapy for the treatment (TX) of metastatic colorectal cancer (MCRC): results of pooled data from 4 clinical studies [abstract no. 359P]. 33rd Congress of the European Society for Medical Oncology; 2008 Sep 12–16; Stockholm
Moroni M, Veronese S, Benvenuti S, et al. Gene copy number for epidermal growth factor receptor (EGFR) and clinical response to antiEGFR treatment in colorectal cancer: a cohort study. Lancet Oncol 2005 May; 6(5): 279–86
Sartore-Bianchi A, Moroni M, Veronese S, et al. Epidermal growth factor receptor gene copy number and clinical outcome of metastatic colorectal cancer treated with panitumumab. J Clin Oncol 2007 Aug 1; 25(22): 3238–45
Di Nicolantonio F, Martini M, Molinari F, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol 2008 Dec 10; 26(35): 5705–12
Sartore-Bianchi A, Martini M, Molinari F, et al. PIK3CA mutations in colorectal cancer are associated with clinical resistance to EGFR-targeted monoclonal antibodies. Cancer Res 2009 Mar 1; 69(5): 1851–7
Carcereny E, Castellvi-Bel S, Alonso V, et al. EGFR polymorphisms as predictors of clinical outcome in patients with advanced colorectal cancer (ACRC) treated with cetuximab and panitumumab [abstract no. 4124]. 44th Annual Meeting of the American Society of Clinical Oncology; 2008 May 30–Jun 2; Chicago (IL)
Perkins G, Lièvre A, Ramacci C, et al. Additional value of EGFR downstream signaling phosphoprotein expression to KRAS status for response to antiEGFR antibodies in colorectal cancer. Int J Cancer. Epub 2010 Jan 4
Siena S, Sartore-Bianchi A, Di Nicolantonio F. Biomarkers predicting clinical outcome of epidermal growth factor receptor-targeted therapy in metastatic colorectal cancer. J Natl Cancer Inst 2009 Oct 7; 101(19): 1308–24
Lacouture ME, Mitchell EP, Piperdi B, et al. Skin toxicity evaluation protocol with panitumumab (STEPP), a phase II, open-label, randomized trial evaluating the impact of a pre-emptive skin treatment regimen on skin toxicities and quality of life in patients with metastatic colorectal cancer. J Clin Oncol 2010 Mar 10; 28(8): 1351–7
Starcevic M, Weeraratne D, Lofgren JA, et al. Panitumumab immunogenicity in two phase III trials of patients with metastatic colorectal cancer (mCRC) treated with panitumumab plus FOLFIRI or FOLFOX4 [abstract no. 433]. 2010 Gastrointestinal Cancers Symposium; 2010 Jan 22–24; Orlando (FL)
Author information
Authors and Affiliations
Corresponding author
Additional information
Adapted and reproduced from Drugs 2010; 70 (8): 1059–78. The full text article[1] was reviewed by A.B. Benson III, Robert H. Lurie Comprehensive Cancer Center of Northwestern University, Chicago, Illinois, USA; J.-L. Canon, Medical Oncology, Grand Hopital de Charleroi, Charleroi, Belgium; J. Cassidy, Cancer Research UK, Glasgow, Scotland; A. Hendlisz, Medical Oncology Clinic, Institut Jules Bordet, Brussels, Belgium; T.J. Price, Department of Oncology, The Queen Elizabeth Hospital and University of Adelaide, Adelaide, South Australia, Australia; W. Scheithauer, Division of Clinical Oncology, Department of Internal Medicine I and Cancer Center, Medical University Vienna, Vienna, Austria; J.-L. Van Laethem, Department of Gastroenterology-GI Cancer Unit, Erasme University Hospital, Brussels, Belgium. The manufacturer of the agent under review was offered an opportunity to comment on the original article during the peer review process. Changes based on any comments received were made on the basis of scientific and editorial merit. The preparation of the original article and this spotlight was not supported by any external funding
Rights and permissions
About this article
Cite this article
Keating, G.M. Spotlight on Panitumumab in Metastatic Colorectal Cancer. BioDrugs 24, 275–278 (2010). https://doi.org/10.2165/11205460-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11205460-000000000-00000