Abstract
Objective: To investigate the dosage form proportionality and food effect of the final tablet formulation of eslicarbazepine acetate (ESL) in healthy volunteers.
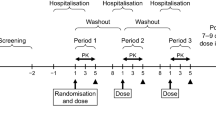
Methods: This was a randomized, three-way crossover, single-centre study in 18 healthy volunteers. Subjects received a single dose of oral ESL 800 mg following a standard meal in one period, and following 10 hours of fasting in two separate periods (in the form of one 800 mg tablet [reference] or two 400 mg tablets [test]). The statistical method was based upon the 90% confidence interval (CI) of maximum observed plasma drug concentration (Cmax), area under the plasma concentration time curve (AUC) from time zero to the last sampling time at which concentrations were at or above the lower limit of quantification (AUCt) and AUC from time zero to infinity (AUC∞) geometric means ratios (GMRs) of BIA 2-005, the enantiomeric mixture of the ESL active metabolite eslicarbazepine and its enantiomer R-licarbazepine. Bioequivalence was assumed when the 90% CI of the test/reference GMR fell within the bioequivalence acceptance interval (80.00, 125.00).
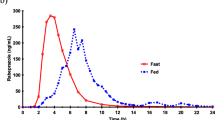
Results: Following a single dose of ESL 800 mg in the forms of two 400 mg tablets and one 800 mg tablet, the test/reference GMR (%) and 90% CI for Cmax, AUCt and AUC∞ were 100.78% (93.91, 108.16), 100.37% (97.82, 102.99) and 100.48% (97.91, 103.13), respectively. Following administration of one 800 mg tablet in fed (test) and fasting (reference) conditions, the test/reference GMR and 90% CI for Cmax, AUCt and AUC8 were 100.96% (94.08, 108.35), 96.79% (94.34, 99.32) and 96.75% (94.27, 99.29), respectively. Treatments were well tolerated.
Conclusions: The bioequivalence criteria between the ESL 400 mg and 800 mg tablets were met and dosage form proportionality was demonstrated. The presence of food had no influence on ESL pharmacokinetics, indicating that ESL can be administered without regard to meals with no significant effects on drug disposition or extent of systemic exposure.




Similar content being viewed by others
References
Shorvon SD. The epidemiology and treatment of chronic and refractory epilepsy. Epilepsia 1996; 37 Suppl. 2: 1–3
Bialer M. New antiepileptic drugs that are second generation to existing antiepileptic drugs. Expert Opin Investig Drugs 2006; 15: 637–47
Committee for Proprietary Medicinal Products, European Agency for the Evaluation of Medicinal Products (EMEA). Note for guidance on clinical investigation of medicinal products in the treatment of epileptic disorders (CPMP/EWP/566/98 rev 1). London: EMEA, 2000 16 Nov
Elger C, Bialer M, Cramer JA, et al. Eslicarbazepine acetate: a double-blind, add-on, placebo-controlled exploratory trial in adult patients with partial-onset seizures. Epilepsia 2007; 48: 497–504
Benes J, Parada A, Figueiredo AA, et al. Anticonvulsant and sodium channel-blocking properties of novel 10,11-dihy-dro-5H-dibenz[b,f]azepine-5-carboxamide derivatives. J Med Chem 1999; 42: 2582–7
Hainzl D, Parada A, Soares-da-Silva P. Metabolism of two new antiepileptic drugs and their principal metabolites S(+)- and R(-)-10,11-dihydro-10-hydroxy carbamazepine. Epilepsy Res 2001; 44: 197–206
Lima R, Vasconcelos T, Cerdeira R, et al. Bioequivalence of final tablet formulation and research tablet formulation of eslicarbazepine acetate in healthy volunteers [poster 857]. 8th European Congress on Epileptology; 2008 Sep 21–25; Berlin
Maia J, Vaz-da-Silva M, Almeida L, et al. Effect of food on the pharmacokinetic profile of eslicarbazepine acetate (BIA 2-093). Drugs R D 2005; 6: 201–6
Almeida L, Soares-da-Silva P. Eslicarbazepine acetate (BIA 2-093). Neurotherapeutics 2007; 4: 88–96
Almeida L, Falcão A, Maia J, et al. Single-dose and steady-state pharmacokinetics of eslicarbazepine acetate (BIA 2-093) in healthy elderly and young subjects. J Clin Pharmacol 2005; 45: 1062–6
Almeida L, Soares-da-Silva P. Safety, tolerability and pharma-cokinetic profile of BIA 2-093, a novel putative antiepileptic agent, during first administration to humans. Drugs R D 2003; 4: 269–84
Almeida L, Soares-da-Silva P. Safety, tolerability, and pharma-cokinetic profile of BIA 2-093, a novel putative antiepileptic, in a rising multiple-dose study in young healthy humans. J Clin Pharmacol 2004; 44: 906–18
Committee for Proprietary Medicinal Products, European Agency for the Evaluation of Medicinal Products (EMEA). Note for guidance on the investigation of bioavailability and bioequiva-lence (CPMP/EWP/1401/98). London: EMEA, 2001
US Food and Drug Administration/Center for Drug Evaluation and Research. Guidance for industry: bioavailability and bio-equivalence studies for orally administered drug products. General considerations. Rockville (MD): US FDA, 2003
Fontes-Ribeiro C, Nunes T, Falcão A, et al. Eslicarbazepine acetate (BIA 2-093): relative bioavailability and bioequiva-lence of 50 mg/mL oral suspension and 200mg and 800mg tablet formulations. Drugs R D 2005; 6: 253–60
Acknowledgements
This study was supported by BIAL (Portela e Ca SA). Teresa Nunes, Teófilo Vasconcelos, Rui Cerdeira, Ricardo Lima, José-Francisco Rocha, Luís Almeida and Patrício Soares-da-Silva are employees of BIAL. Amílcar Falcão received a consultancy fee from Portela e Ca SA for work on this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fontes-Ribeiro, C., Macedo, T., Nunes, T. et al. Dosage Form Proportionality and Food Effect of the Final Tablet Formulation of Eslicarbazepine Acetate. Drugs in R D 9, 447–454 (2008). https://doi.org/10.2165/0126839-200809060-00007
Published:
Issue Date:
DOI: https://doi.org/10.2165/0126839-200809060-00007