Abstract
Background: A calcipotriol (calcipotriene)/betamethasone dipropionate two-compound product has been shown to be efficacious for the treatment of psoriasis vulgaris. It is usually administered once daily for up to 4 weeks followed by treatment with corticosteroid-free conventional products (e.g. calcipotriol). The aim of this study was to evaluate the efficacy and tolerability of a 4-week treatment with the two-compound product in psoriasis vulgaris and the effects of sequential 8-week maintenance treatment with different calcipotriol formulations.
Methods: After an initial 4-week phase with the once-daily calcipotriol/betamethasone dipropionate two-compound product, adult patients with stable psoriasis vulgaris entered an 8-week maintenance phase and were allocated to one of the following treatments: calcipotriol ointment twice daily, calcipotriol cream twice daily, or calcipotriol cream once daily in the morning and calcipotriol ointment once daily in the evening. Clinical assessment was performed at baseline, after 4 weeks and after 12 weeks, and employed evaluation of the severity of pruritus using a scale from zero to four and the Psoriasis Area and Severity Index (PASI).
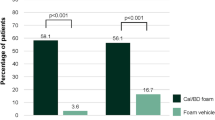
Results: After 4 weeks’ treatment with the calcipotriol/betamethasone dipropionate two-compound product, a significant improvement in the severity of psoriasis was observed in all groups, with a mean reduction in the PASI of 71.3% (p < 0.001 vs baseline). A significant improvement in pruritus was also obtained after 4 weeks. These results were maintained after 8 weeks of treatment with calcipotriol, regardless of the formulation used. Treatment was very well tolerated and accepted by patients. On a scale ranging from poor to excellent, more patients treated with calcipotriol cream (49%) rated the acceptability of the treatment as excellent when compared with patients treated with the calcipotriol ointment (33%) or both calcipotriol formulations (36%).
Conclusion: This study shows that the calcipotriol/betamethasone dipropionate two-compound product causes a rapid and marked improvement in both psoriasis lesions and pruritus. Our preliminary results suggest that the three calcipotriol-based regimens are equally effective in maintaining the therapeutic results obtained with the calcipotriol/betamethasone dipropionate two-compound product and that the use of calcipotriol cream was the best accepted maintenance treatment.




Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement. Dovobet®, Daivobet® is marketed as Taclonex® in the US.
References
Lange K, Kleuser B, Gysler A, et al. Cutaneous inflammation and proliferation in vitro: differential effects and mode of action of topical glucocorticoids. Skin Pharmacol Appl Skin Physiol 2000; 13: 93–103
Mozzanica N, Cattaneo A, Schmitt E, et al. Topical calcipotriol for psoriasis: an immunohistologic study. Acta Derm Venereol Suppl 1994; 186: 171–2
Lu I, Gilleaudeau P, McLane JA, et al. Modulation of epidermal differentiation, tissue inflammation, and T-lymphocyte infiltration in psoriatic plaques by topical calcitriol. J Cutan Pathol 1996; 23: 419–30
Jensen AM, Llado MB, Skov L, et al. Calcipotriol inhibits the proliferation of hyperproliferative CD29 positive keratinocytes in psoriatic epidermis in the absence of an effect on the function and number of antigen-presenting cells. Br J Dermatol 1998; 139: 984–91
Fenton C, Plosker GL. Calcipotriol/betamethasone dipropionate: a review of its use in the treatment of psoriasis vulgaris. Am J Clin Dermatol 2004; 5: 463–78
van de Kerkhof PC, Wasel N, Kragballe K, et al. A two-compound product containing calcipotriol and betamethasone dipropionate provides rapid, effective treatment of psoriasis vulgaris regardless of baseline disease severity. Dermatology 2005; 210: 294–9
Kragballe K, Noerrelund KL, Lui H, et al. Efficacy of once-daily treatment regimens with calcipotriol/betamethasone dipropionate ointment and calcipotriol ointment in psoriasis vulgaris. Br J Dermatol 2004; 150: 1167–73
Ortonne JP, Kaufmann R, Lecha M, et al. Efficacy of treatment with calcipotriol/betamethasone dipropionate followed by calcipotriol alone compared with tacalcitol for the treatment of psoriasis vulgaris: a randomised, double-blind trial. Dermatology 2004; 209: 308–13
Fredriksson T, Pettersson U. Severe psoriasis: oral therapy with a new retinoid. Dermatologica 1978; 157: 238–44
Kaufmann R, Bibby AJ, Bissonnette R, et al. A new calcipotriol/betamethasone dipropionate formulation (Daivobet) is an effective once-daily treatment for psoriasis vulgaris. Dermatology 2002; 205: 389–93
Guenther L, van de Kerkhof PC, Snellman E, et al. Efficacy and safety of a new combination of calcipotriol and betamethasone dipropionate (once or twice daily) compared to calcipotriol (twice daily) in the treatment of psoriasis vulgaris: a randomized, double-blind, vehicle-controlled clinical trial. Br J Dermatol 2002; 147: 316–23
Douglas WS, Poulin Y, Decroix J, et al. A new calcipotriol/betamethasone formulation with rapid onset of action was superior to monotherapy with betamethasone dipropionate or calcipotriol in psoriasis vulgaris. Acta Derm Venereol 2002; 82: 131–5
Papp KA, Guenther L, Boyden B, et al. Early onset of action and efficacy of a combination of calcipotriene and betamethasone dipropionate in the treatment of psoriasis. J Am Acad Dermatol 2003; 48: 48–54
Traulsen J. Bioavailability of betamethasone dipropionate when combined with calcipotriol. Int J Dermatol 2004; 43: 611–7
van Rossum MM, van Erp PE, van de Kerkhof PC. Treatment of psoriasis with a new combination of calcipotriol and betamethasone dipropionate: a flow cytometric study. Dermatology 2001; 203: 148–52
Vissers WH, Berends M, Muys L, et al. The effect of the combination of calcipotriol and betamethasone dipropionate versus both monotherapies on epidermal proliferation, keratinization and T-cell subsets in chronic plaque psoriasis. Exp Dermatol 2004; 13: 106–12
van De Kerkhof PC. The impact of a two-compound product containing calcipotriol and betamethasone dipropionate (Daivobet/Dovobet) on the quality of life in patients with psoriasis vulgaris: a randomized controlled trial. Br J Dermatol 2004; 151: 663–8
Ruzicka T, Lorenz B. Comparison of calcipotriol monotherapy and a combination of calcipotriol and betamethasone valerate after 2 weeks’ treatment with calcipotriol in the topical therapy of psoriasis vulgaris: a multicentre, double-blind, randomized study. Br J Dermatol 1998; 138: 254–8
Kragballe K, Barnes L, Hamberg KJ, et al. Calcipotriol cream with or without concurrent topical corticosteroid in psoriasis: tolerability and efficacy. Br J Dermatol 1998; 139: 649–54
Yosipovitch G, Goon A, Wee J, et al. The prevalence and clinical characteristics of pruritus among patients with extensive psoriasis. Br J Dermatol 2000; 143: 969–73
Sampogna F, Gisondi P, Melchi CF, et al. Prevalence of symptoms experienced by patients with different clinical types of psoriasis. Br J Dermatol 2004; 151: 594–9
Scott LJ, Dunn CJ, Goa KL. Calcipotriol ointment: a review of its use in the management of psoriasis. Am J Clin Dermatol 2001; 2: 95–120
Kragballe K, Barnes L, Hamberg KJ, et al. Calcipotriol cream with or without concurrent topical corticosteroid in psoriasis: tolerability and efficacy. Br J Dermatol 1998; 139: 649–54
Acknowledgements
No external sources of funding were used in conducting this study, and the authors have no conflicts of interest that are directly relevant to the contents of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cassano, N., Miracapillo, A., Coviello, C. et al. Treatment of Psoriasis Vulgaris with the Two-Compound Product Calcipotriol/Betamethasone Dipropionate followed by Different Formulations of Calcipotriol. Clin. Drug Investig. 26, 227–233 (2006). https://doi.org/10.2165/00044011-200626040-00008
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-200626040-00008