Abstract
Objective: Patients undergoing total joint replacement procedures have a remarkably high incidence of postoperative nausea and vomiting. The purpose of this study was to evaluate the efficacy and safety of ramosetron, a serotonin 5-HT3 receptor antagonist, for the prevention of nausea and vomiting after total hip replacement.
Design: Prospective, randomised, double-blind, placebo-controlled study.
Setting: University-affiliated teaching hospital.
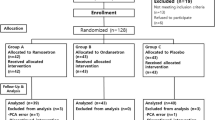
Patients: Eighty patients, aged 35–77 years, 22 male and 58 female, scheduled for total hip replacement.
Interventions: Patients received in a randomised, double-blind manner intravenously administered placebo or ramosetron in three different doses (0.15, 0.3 or 0.6mg) at the completion of surgery (n = 20 in each group). A standard general anaesthetic technique and postoperative analgesia were used.
Main outcome measures and results: Emetic episodes were recorded and safety assessments performed over 0 to 24 hours after anaesthesia. The rate of patients experiencing emetic symptoms (nausea, retching, vomiting) over 0 to 24 hours after anaesthesia was 70% with ramosetron 0.15mg (p = 0.5), 25% with ramosetron 0.3mg (p = 0.002) and 20% with ramosetron 0.6mg (p = 0.001), compared with placebo (75%). No clinically important adverse events were observed in any of the groups.
Conclusion: Prophylactic antiemetic therapy with ramosetron 0.3mg is efficacious against postoperative nausea and vomiting 0 to 24 hours after anaesthesia in patients undergoing total hip replacement. Increasing the dose to 0.6mg provided no further benefit.


Similar content being viewed by others
References
Gan TJ, Alexander R, Fennelly M, et al. Comparison of different methods of administering droperidol in patient-controlled analgesia in the prevention of postoperative nausea and vomiting. Anesth Analg 1995; 80: 81–5
Kauste A, Tuominen M, Heikkinen H, et al. Droperidol, alizapride and metoclopramide in the prevention and treatment of post-operative emetic sequelae. Eur J Anaesthesiol 1986; 3: 1–9
Watcha MF, White PF. Postoperative nausea and vomiting: its etiology, treatment, and prevention. Anesthesiology 1992; 77: 161–84
Gan TJ, Collis R, Hetreed M. Double-blind comparison of ondansetron, droperidol and saline in the prevention of postoperative nausea and vomiting. Br J Anaesth 1994; 72: 544–7
Fijihara A, Akuzawa S, Miyata K, et al. Ramosetron hydrochloride: affinity for cloned human 5-HT3 receptor antagonistic and antiemetic effect in ferret [in Japanese]. Laboratory and Clinic 1996; 30: 1965–72
Noda K, Ikeda M, Yoshida O, et al. Clinical evaluation of ramosetron against the nausea and vomiting induced by anticancer drugs [in Japanese]. Jpn J Clin Exp Med 1994; 71: 2765–76
Kang Y-K, Park YH, Ryoo B-Y, et al. Ramosetron for the prevention of cisplatin-induced acute emesis: a prospective randomized comparison with granisetron. J Int Med Res 2002; 30: 220–9
Kamato T, Ito H, Nagakura Y, et al. Mechanism of cisplatin- and m-chlorophenylbiguanide-induced emesis in ferret. Eur J Pharmacol 1993; 238: 369–76
Kawabata Y, Sakiyama H, Muto S, et al. Clinical evaluation and pharmacokinetics of ramosetron against the nausea and vomiting induced by anticancer drugs [in Japanese]. Nishinihon J Urol 1994; 56: 1445–56
Acknowledgements
No sources of funding were used to assist in the preparation of this manuscript. The authors have no conflicts of interest that are directly relevant to the content of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article has been retracted by the journal after an investigation found the data to be fabricated.
An erratum to this article can be found online at http://dx.doi.org/10.1007/s40261-014-0189-y.
About this article
Cite this article
Fujii, Y., Tanaka, H. RETRACTED ARTICLE: Prevention of Nausea and Vomiting with Ramosetron after Total Hip Replacement. Clin. Drug Investig. 23, 405–409 (2003). https://doi.org/10.2165/00044011-200323060-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-200323060-00004