Abstract
Objective
To determine the urinary excretion of N-acetyl-β-D-glucosaminidase (NAG; early index of renal proximal tubular damage) and epidermal growth factor (EGF; early index of renal damage repair) in paediatric patients with and without vesico-ureteric reflux (VUR) receiving prophylactic cefixime for recurrent urinary tract infections (UTIs).
Design and Setting
Urinary levels of NAG and EGF in children, with and without VUR, with recurrent UTIs receiving prophylactic cefixime were compared with normal paediatric laboratory values in a university paediatric department. All children were followed during an ordinary admission or in a day hospital.
Participants and Treatment
The study population consisted of 27 patients (15 males, 12 females; mean age 1.73 ± 1.43 years) followed in the Paediatric Department of the University of Verona for recurrent UTIs. All patients had experienced at least two episodes of UTIs in the previous 2 months. Patients received antibiotic prophylaxis with cefixime (4 mg/kg bodyweight), administered as a single bedtime dose. The overall duration of the treatment ranged from 1 to 2 months. Urine samples and cultures were taken immediately prior to voiding urethrocystography via the bladder catheter and were immediately frozen at —20°C. NAG activity and EGF levels in the urine were determined using a colorimetric assay and a radioimmunoassay, respectively. The urinary creatinine level was determined using the Jaffe kinetic colorimetric method at a constant reaction temperature of 37°C. The values obtained were compared with the Laboratory’s own reference standards for paediatric patients.
Main Outcome Measures and Results
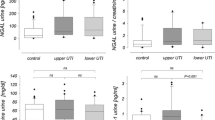
All children in the study population presented with normal routine laboratory values, in particular serum creatinine and BUN levels. In addition, urine tests and cultures yielded normal values in all cases. In patients without VUR receiving prophylactic cefixime, the mean [±standard deviation (SD)] NAG level was 0.50 ± 0.30 U/mmol creatinine (range0.05 to 1.17). Only one of 20 patients had a slight increase above the normal range. In patients with VUR, the urinary NAG level was 2.55 ± 1.66 U/mmol creatinine (range 1.37 to 6), with all seven patients having abnormally elevated NAG values. The difference in NAG levels between the two groups was statistically significant (p < 0.001). In patients receiving prophylactic cefixime without VUR (group 1), the mean urinary EGF level was 25.06 ± 16.05 μg/L (range 1.58 to 49.63). In patients receiving prophylaxis with cefixime with VUR treatment (group 2), the mean (± SD) urinary EGF level was 38.23 ± 33.99 μg/L (range 14.4 to 107). Urinary EGF levels were not statistically different between group 1 and normal levels, whereas in group 2, EGF levels were significantly higher than normal levels or those of group 1 (p < 0.05, both comparisons).
Conclusions
Prophylactic treatment with cefixime was well tolerated, including renally, in children with recurrent UTIs without VUR. Renal tolerability was assessed using a sensitive measure of the early signs of nephrotoxicity, such as urinary NAG values, and markers of damage repair, such as urinary EGF levels. The presence of VUR in patients was associated with significant renal proximal tubular damage, as reflected in elevated urinary NAG values. The high urinary EGF values probably reflect the compensatory repair action of the kidney in patients with VUR. In conclusion, in clinical practice, cefixime may be safely suggested for antibiotic prophylaxis in paediatric patients with recurrent UTIs.


Similar content being viewed by others
References
Zelicovich I, Adelman RD, Nancarrow PA. Urinary tract infections in children. An update. West J Med 1992; 157: 554–61
Fanos V, Agostiniani R, Cataldi L. Pyelectasis and hydro-nephrosis in the newborn and infant. Acta Pediatr 2000; 89: 200–4
Quinet B. Définition actuel de l’infection urinaire de l’enfant. Arch Pediat 1998; Suppl. 3: 250–3
Bitar CN, Steele RW. Use of prophylactic antibiotics in children. Adv Pediatr Infect Dis 1995; 10: 227–62
Mangiarotti P, Pizzini C, Fanos V. Antibiotic prophylaxis in children with relapsing urinary tract infections. J Chemother 2000; 12(2): 115–23
Fanos V, Dall’Agnola A. Antibiotics in neonatal infections: a review. Drugs 1999, 58(3): 406–27
Fanos V, Cataldi L. Antibacterial induced nephrotoxicity in the newborn. Drug Safety 1999; 20(3): 245–67
Brittain DC, Scull BE, Hirose T, et al. The pharmacokinetic and bactericidal characteristics of oral cefixime. Clin Pharmacol Ther 1985; 38(5): 590–4
Barry AL, Jones RN. Cefixime: spectrum of antibacterial activity against 16016 clinical isolates. Pediatr Infect Dis J 1987; 6: 954–7
Brodgen RN, Campoli-Richards DM. Cefixime: a review of its antibacterial activity, pharmacokinetic properties and therapeutic potential. Drugs 1989; 38: 524–50
Neu HC. In vitro activity of a new broad spectrum, beta-lactamase-stable oral cephalosporin, cefixime. Pediatr Infect Dis J 1987; 6: 954–7
Tally FP, Cartwright K, Desjardins RE, et al. Safety profile of cefixime. Pediatr Infect Dis J 1987; 6: 976–80
Mussap M, Fanos V, Cataldi L, et al. Urinary low molecular mass proteins and enzymes as early, non invasive markers of nephrotoxicity in the neonate. Eur J Lab Med 1998; 6(1): 1–14)
Savage CR Jr, Inagami T, Cohen S. The primary structure of epidermal growth factor. J Biol Chem 1972; 247: 7612–21
Savage CR Jr, Hash JH, Cohen S. Epidermal growth factor, location of disulfide bonds. J Biol Chem 1973; 248: 7669–72
Hofmann GE, Abramowicz JS. Epidermal growth factor (EGF) concentrations in amniotic fluid and maternal urine during pregnancy. Acta Obstet Gynecol Scand 1990; 69(3): 217–21
Mattila AL, Perheemtupa J, Pesonen K, et al. Epidermal growth factor in human urine from birth to puberty. J Clin Endocrinol Metab 1985; 61: 997–1000
Stoll DM, King LE Jr, McNeil L, et al. Human urinary epidermal growth factor excretion: age, sex, and race dependence. J Clin Endocrinol Metab 1988; 67: 361–7
Hirata Y, Moore GW, Bertagna C, et al. Plasma concentration of immunoreactive human epidermal growth factor (uro-gastrone) in man. J Clin Endocrinol Metab 1980; 50: 440–4
Dagogo-Jack S. Epidermal growth factor EGF in human saliva: effect of age, sex, race, pregnancy and sialogogue. Scand J Gastroenterol 1986; 124 Suppl.: 47–54
Plebani M, Fanos V, Mussap M, et al. Urinary excretion of human epidermal growth factor in premature infants requiring assisted ventilation over the first week of life. Nephron 1997; 76: 225–6
Callegari C, Laborde NP, Buenaflor G, et al. The source of urinary epidermal growth factor in humans. Eur J Appl Physiol 1988; 58: 26–31
Salido EC, Lakshmanan J, Fisher DA, et al. Immunocyto-chemical localization of epidermal growth factor prohormone in adult human kidney. Clin Res 1991; 39: 112A
Humes HD, Cieslinski DA, Coimbra TM, et al. Epidermal growth factor enhances renal tubule cell regeneration and repair and accelerates the recovery of renal function in postischemic acute renal failure. J Clin Invest 1989; 84: 1757–61
Kanda S, Saha PK, Nomata K, et al. Transient increase in renal epidermal growth factor content after unilateral nephrectomy in the mouse. Acta Endocrinol Copenh 1991 Feb; 124(2): 188–93
Vinter Jensen L, Smerup M, Jørgensen PE, et al. Chronic treatment with epidermal growth factor stimulates growth of the urinary tract in the rat. Urol Res 1996; 24: 15–21
Csàthy L, Pòcsi I. Urinary N-acetyl-β-D-glucosaminidase determination in newborns and children: methods and diagnostic applications. Eur J Clin Chem Clin Biochem 1995; 33: 575–87
Olah VA, Csàthy L, Varga J, et al. Reference ranges for urinary N-acetyl-β-D-glucosaminidase in healthy children determined with three colorimetric methods. Ann Clin Biochem 1994; 31: 87–8
Neu HC, Chin NX, Labthavikul P. Comparative in vitro activity and β-lactamase stability of FR-17027, a new orally active cephalosporin. Antimicrob Agents Chemother 1984; 26: 174–80
Pennie RA. Cefixime in pediatric infections. In: Adam D, Quintiliani R, editors. Cefixime. Torre Lazur: McCann 2000: 79–83
Dagan R, Einhorn M, Lang R, et al. Once daily cefixime compared with twice daily trimethoprim/sulfamethoxazole for treatment of urinary tract infection in infants and children. Pediatr Infect Dis J 1992; 11: 98–203
Pizzini C, Mussap P, Mangiarotti P, et al. Urinary biomarkers in children with and without reflux on antibiotic prophylaxis with cefaclor. Clin Drug Invest 1999; 18(6): 461–6
Fanos V, Agostiniani R, Cataldi L. Vesicoureteric reflux: an update. Pediatr Med Chir 1999; 21(2): 47–56
Cunha BA. Third generation cephalosporines: a review. Clin Ther 1992; 14: 616–52
Norrby SR. Adverse reactions and interactions with newer cephalosporins and cephamycin antibiotics. Med Toxicol 1986; 1: 32–46
Tune BM. Renal tubular transport and nephrotoxicity of beta-lactam antibiotics: structure-activity relationship. Miner Electrolyte Metab 1994; 20: 221–31
Tune BM. Nephrotoxicity of beta-lactam antibiotics: mechanism and strategies for prevention. Pediatr Nephrol 1997; 11(6): 768–72
Kaloyanides GJ. Antibiotic related nephrotoxicity. Nephrol Dial Transplant 1994; 9(4 Suppl.): 130S–40S
Morin JP, Fillastre JP, Vaillant R. In vitro effects of amino-glycosides and cephalosporins on rat kidney lysosomes. C R Seances Soc Biol Fil 1977; 171(5): 1088–93
Naruse T, Hirokawa N, Oike S. Clinical evaluation of urinary N-acetyl-beta-D-glucosaminidase activity in patients receiving aminoglycoside and cephalosporin drugs. Res Commun Chem Pathol Pharmacol 1981; 31(2): 313–29
Furukawa S, Okada T. Clinical experience with cefixime in pediatric infections (in Japanese). Jpn J Antibiot 1986; 39(4): 1128–37
Mikawa H, Mayumi M, Akiyama Y, et al. Clinical studies on cefixime in pediatrics [in Japanese]. Jpn J Antibiot 1989 Dec; 42(12): 2527–39
Risser WL, Barone JS, Clark PA, et al. Noncomparative, open label, multicenter trial of cefixime for treatment of bacterial pharyngitis, cystitis and pneumonia in pediatric patients. Pediatr Infect Dis J 1987 Oct; 6(10): 1002–6
Tally FP, Cartwright K, Desjardins RE, et al. Safety profile of cefixime. Pediatr Infect Dis J 1987; 6: 976–80
Garcia Rodriguez JA, Munoz Bellido JL. Therapeutic use of cefixime in urinary tract infections infections. In: Adam D, Quintiliani R, editors. Cefixime. Torre Lazur: McCann, 2000: 95–101
Fanos V, Cataldi L. Cefixime in urinary tract infections with particular reference to pediatrics. J Chemother 2001; 13(2): 112–7
Fanos V, Pizzini C, Mussap M, et al. Urinary epidermal growth factor in different renal conditions. Ren Fail. In press
Cuzzolin L, Mangiarotti P, Fanos V. Urinary PGE2 concentrations measured by a new EIA method in infants with urinary tract infections or renal malformations. Prostaglandins Leukot Essent Fatty Acids. In press
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fanos, V., Mussap, M., Osio, D. et al. Urinary Excretion of N-Acetyl-β-D-Glucosaminidase and Epidermal Growth Factor in Paediatric Patients Receiving Cefixime Prophylaxis for Recurrent Urinary Tract Infections. Clin. Drug Investig. 21, 511–518 (2001). https://doi.org/10.2165/00044011-200121070-00007
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-200121070-00007