Abstract
Objective
This study aimed to investigate the relative bioavailability and bioequivalence of two omeprazole enteric-coated formulations following repeated doses (steady state) in healthy male and female adult volunteers.
Design and Study Participants
The study formulation (Ompranyt® 20mg capsules, Bial-Industrial Farmaceutica SA, Spain) was compared with an omeprazole reference formulation (Mopral® 20mg capsules, Laboratório Astra, Spain). 24 participants were randomised using a two-way, crossover design to receive either one capsule/day of Ompranyt® or one capsule/day of Mopral® during two sequential periods of five consecutive days each. The participants were administered the drugs in the fasting state. Omeprazole concentrations in plasma samples were quantified by a validated method using a reversed-phase high performance liquid chromatography with UV detection (HPLC-UV). The validation method is described.
Setting
The study was conducted at the Human Pharmacology Unit, Department of Research & Development, Laboratorios Bial (S. Mamede do Coronado, Portugal).
Results
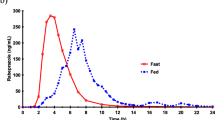
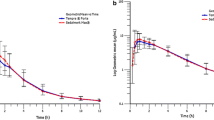
The arithmetic mean ± SD values of the area under the plasma concentration versus time curve from time zero to infinity (AUC0-∞) were 1474 ± 1417 μg/L·h for Ompranyt® and 1490 ± 1276 μg/L·h for Mopral®. The geometric means ratio (Ompranyt®/Mopral®) was 0.99, with 90% confidence intervals (CI) of 0.97–1.03. The estimated maximum plasma concentration (Cmax) was 630.1 ± 516.7 μg/L for Ompranyt® and 736.7 ± 443.3 μg/L for Mopral®, with a geometric means ratio (Ompranyt®/Mopral®) of 0.96 (90% CI: 0.94-0.99). Bioequivalence of these two formulations was accepted based on the two one-sided ANOVA for AUC0-∞ as well as for Cmax. In both cases, the 90% CI lies within the acceptance range of 0.80–1.25.
Conclusion
Bioequivalence of Ompranyt® and Mopral® was demonstrated after repeated drug administration in fasting conditions, and both products were similarly well tolerated. Therefore, both formulations are expected to be equivalent in a clinical setting.




Similar content being viewed by others
References
Howden CW. Clinical pharmacology of omeprazole. Clin Pharmacokinet 1991; 20: 38–49
AHFS (American Hospital Formulary Service) Drug Information. Omeprazole. Bethesda, United States of America: American Society of Health-System Pharmacists, 1999
Howden CW, Meredith PA, Forrest JAH, et al. Oral pharmacokinetics of omeprazole. Eur J Clin Pharmacol 1984; 26: 641–3
Prichard PJ, Yeomans ND, Mihaly CW, et al. Omeprazole: a study of its inhibition of gastric pH and oral pharmacokinetics after morning or evening dosage. Gastroenterology 1985; 88: 64–9
Richards JP, Gimeno M, Moreland TA, et al. Estudio de biodisponibilidad relativa de dos formulaciones orales de omeprazol tras la administratción en dosis repetidas a voluntarios sanos. Gastroenterol Hepatol 1999; 22: 171–82
USP-DI (United States Pharmacopoeia — drug information). 19th ed. Omeprazole. Englewood, United States of America: Micromedex, 1999
Farinha A, Bica A, Pais JP, et al. Bioequivalence evaluation of two omeprazole enteric-coated formulations in humans. Eur J Pharmaceutical sci 1999; 7: 311–5
Garg SK, Chugh Y, Tripathi SK, et al. Comparative bioavailability of two enteric-coated capsules of omeprazole in healthy volunteers. Int J Clin Pharmacol Ther Toxicol 1993; 31(2): 96–9
Pillai GK, Salem MS, Najib NM, et al. Bioequivalence study of two capsule formulations of omeprazole. Acta Pharmaceutica Hungarica 1996; 66: 231–5
Steinijans VW, Sauter S, Hauschke D, et al. Reference tables for the intrasubject coefficient of variation in bioequivalence studies [short communication]. Int J Clin Pharmacol Ther 1995; 33: 427–30
CPMP/EWP/QWP/1401/98. Note for guidance on the investigation of bioavailability and bioequivalence. European Agency for the Evaluation of Medicinal Products (EMEA), Committee for Proprietary Medicinal Products (CPMP), London, December 2000. Relative bioavailability of two enteric-coated formulations of omeprazole following repeated doses in healthy volunteers
Acknowledgements
This study was financially supported by Laboratorios Bial, Portugal.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vaz-da-Silva, M., Hainzl, D., Almeida, L. et al. Relative Bioavailability of Two Enteric-Coated Formulations of Omeprazole following Repeated Doses in Healthy Volunteers. Clin. Drug Investig. 21, 203–210 (2001). https://doi.org/10.2165/00044011-200121030-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-200121030-00006