Summary
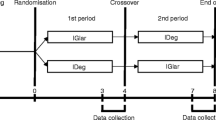
Fifteen patients with insulin-dependent diabetes mellitus who were stabilised on insulin, participated in an open study to assess the short- and long-term effects of ipsapirone on plasma glucose profiles, and to collect pharmacokinetic data on ipsapirone in this patient population. Plasma glucose concentrations were determined on day 1 (baseline), day 2 (after a single dose of ipsapirone HCl 5mg) and day 7 (after multiple doses of ipsapirone HCl 5mg three times daily) of the study. Plasma ipsapirone concentrations were determined on days 2 and 7. For ethical reasons, a crossover design could not be used, and the treatment order was the same for all the patients. Consequently, the effect of treatment (ipsapirone) on plasma glucose concentrations and possible period effects were confounded. Analysis of covariance and correlation analysis were used to assess the effect of ipsapirone on plasma glucose concentrations. The geometric means (SD) of the plasma glucose AUC0–5h on days 1, 2 and 7 were 56.9 (1.44), 48.1 (1.60) and 44.7 (1.48) mmol/L·h, respectively. The corresponding geometric mean (SD) trough (Oh) plasma glucose concentrations were 10.0 (1.58), 7.21 (1.96) and 6.69 (1.79) mmol/L. Correction for differences in trough glucose concentrations by an analysis of covariance indicated that a single dose of ipsapirone HC1 does not influence plasma glucose concentrations. Lack of correlation between the individual ipsapirone AUCss 0–8h on day 7, and the difference between days 1 and 7 in glucose AUC0–5h, indicated that this also applies to multiple doses of ipsapirone. These results suggest that the addition of ipsapirone to insulin regimens does not affect plasma glucose levels to such an extent that an adjustment of insulin dosage is required. Plasma ipsapirone concentrations decline biphasically, with median half-lives of 0.24 hours and 1.71 hours after a single dose, and median half-lives of 0.25 hours and 2.03 hours after multiple doses. The maximum concentrations after a single dose are similar to those after multiple doses, but the AUCss 0–8h is somewhat higher than the AUC0–∞ (after a single dose), indicating slight accumulation of the drug.
Similar content being viewed by others
References
Lucki I. Behavioral studies of serotonin receptor agonists as antidepressant drugs. J Clin Psychiatry 1991; 52: 24–31
Brockmeier D, Lückel G. HOEREP-PC Version (1.05.00). An interactive program package for the analysis of pharmacokinetic data. User manual. International report, Document No.: 011502, Hoechst AG, Frankfurt/Main (1991)
Brockmeier D. In vitro/in vivo correlation of dissolution using moments of dissolution and transit times. Acta Pharm Technol 1986; 32: 164–74
Pfeffer M. Estimation of mean residence time from data obtained when multiple-dosing steady state has been reached. J Pharm Sci 1984; 73: 854–6
Gardner MJ, Altman DG. Confidence intervals rather than P values: estimation rather than hypothesis testing. BMJ 1986; 292: 746–50
Steinijans VW, Diletti E. Statistical analysis of bioavailability studies: parametric and nonparametric confidence intervals. Eur J Clin Pharmacol 1983; 24: 127–36
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Luus, H.G., Müller, F.O., Groenewoud, G. et al. Effect of Ipsapirone on Plasma Glucose and Pharmacokinetics of Ipsapirone in Type I Diabetics. Clin. Drug Invest. 11, 159–166 (1996). https://doi.org/10.2165/00044011-199611030-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-199611030-00006