Abstract
▲ The new formulation of subcutaneous interferon-β-1a was developed without serum-derived components with the aim of improving immunogenicity and injection tolerability in patients with relapsing forms of multiple sclerosis (MS).
▲ In a prospectively defined interim analysis at 48 weeks of an ongoing, single-arm, phase IIIb trial, 13.9% of MS patients receiving the new formulation of subcutaneous interferon-β-1a 44µg three times weekly had developed neutralising antibodies (NAbs). In the EVIDENCE trial, which served as an historical control, 24.4% of patients receiving the same dosage of the current formulation had developed NAbs at 48 weeks.
▲ The new formulation demonstrated similar pharmacokinetic activity to that of the current formulation in a phase I, double-blind, placebo-controlled study in healthy volunteers.
▲ About two-thirds of patients with MS who received the new formulation of subcutaneous interferon-β-1a were relapse free in the interim, 48-week analysis of the single-arm trial; this is similar to results for the current formulation from historical data.
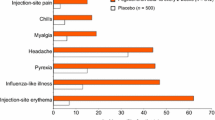
▲ A comparison of results from the interim, 48-week analysis with historical-control data from the EVIDENCE trial indicates that the new formulation of interferon-β-1a may be associated with a lower incidence of injection-site reactions and a higher incidence of influenza-like symptoms than the current formulation.
▲ Adverse events associated with the new formulation were mostly mild to moderate in severity.

Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Noseworthy JH, Lucchinetti C, Rodrigues M, et al. Multiple sclerosis. N Engl J Med 2000; 343(13): 938–52
National Multiple Sclerosis Society. Research/clinical update. National MS Society raises concerns that recent NIH study underestimates number of people with MS in the US [online]. Available from URL: http://www.nationalmssociety.org [Accessed 2007 Jul 4]
O’Connor P. Key issues in the diagnosis and treatment of multiple sclerosis: an overview. Neurology 2002; 59Suppl. 3: S1–33
Murdoch D, Lyseng-Williamson KA. Subcutaneous recombinant interferon-β-1a (Rebif®): a review of its use in relapsing-remitting multiple sclerosis. Drugs 2005; 65(9): 1295–312
Freedman MS. Disease-modifying drugs for multiple sclerosis: current and future aspects. Expert Opin Pharmacother 2006; 7Suppl. 1: S1–9
Refib® patient exposure. Geneva: Merck Serono International SA, 2007 Jul 13. (Data on file)
PRISMS (atPrevention of Relapses and Disability by Interferon β-1a Subcutaneously in Multiple Sclerosis). Randomised double-blind placebo-controlled study of interferon β-1a in relapsing/remitting multiple sclerosis. Lancet 1998; 352(7): 1498–504
Panitch H, Goodin DS, Francis G, et al. Randomized, comparative study of interferon β-1a treatment regimens in MS: the EVIDENCE trial. Neurology 2002; 59: 1496–506
SPECTRIMS Study Group. Randomized controlled trial of interferon-beta-1a in secondary progressive MS: clinical results. Neurology 2001; 56: 1496–504
Gneiss C, Tripp P, Reichartseder F, et al. Differing immunogenic potentials of interferon beta preparations in multiple sclerosis patients. Mult Scler 2006 Dec; 12(6): 731–7
Goodin DS, Frohman EM, Hurwitz B, et al. Neutralizing antibodies to interferon beta: assessment of their clinical and radiographic impact: an evidence report. Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2007; 68(13): 977–84
Jaber A, Baker M. Assessment of the immunogenicity of different interferon beta-1a formulations using ex vivo T-cell assays. J Pharm Biomed Anal 2007; 43: 1256–61
Francis GS, Rice GP, Alsop JC. Interferon β-1a in MS: results following development of neutralizing antibodies in PRISMS. Neurology 2005; 65(1): 48–55
Galetta SL, Markowitz C. US FDA-approved disease-modifying treatments for multiple sclerosis: review of adverse effect profiles. CNS Drugs 2005; 19(3): 239–52
Giovannoni G, Barbarash O, Casset-Semanaz F, et al. Immunogenicity and tolerability of an investigational formulation of interferon-β1a: 24- and 48-week interim analyses of a 2-year, single-arm, historically controlled, phase IIIb study in adults with multiple sclerosis. Clin Ther 2007 Jun; 29(6): 1128–45
Wagstaff AJ, Goa KL. Recombinant Interferon-β-1a: a review of its therapeutic efficacy in relapsing-remitting multiple sclerosis. BioDrugs 1998; 10(6): 471–94
Rebif®: summary of product characteristics. London: Serono Europe Limited, 2003
Bellomi F, Muto A, Palmieri G, et al. Immunogenicity comparison of interferon beta-1a preparations using the BALB/c mouse model: assessment of a new formulation for use in multiple sclerosis. New Microbiol 2007; 30(3): 241–6
Brearley C, Jaber A, Bertolino M, et al. Assessment of the safety, tolerability, and PK/PD properties of two new formulations of subcutaneously administered IFN-β1a: a double-blind, placebo-controlled comparison with the currently available formulation. Int J Clin Pharmacol Ther 2007; 45(6): 307–18
The PRISMS (Prevention of Relapses and Disability by Interferon β-1a Subcutaneously in Multiple Sclerosis) Study Group. PRISMS-4: long-term efficacy of interferon-β-1a in relapsing MS. Neurology 2001; 56: 1628–36
Kappos L, Traboulsee A, Constantinescu C, et al. Long-term subcutaneous interferon beta-1a therapy in patients with relapsing-remitting MS. Neurology 2006; 67(6): 944–53
Scanzillo J, Bennett R, Biancucci P, et al. Product enhancements decrease the incidence of injection site reactions and pain resulting in improved adherence to therapy in patients with multiple sclerosis [abstract plus poster]. 17th Annual Meeting of the European Neurological Society; 2007 Jun 16–20; Rhodes
Merck Serono. New formulation of multiple sclerosis treatment Rebif® approved in European Union (media release), 2007 Aug 30 [online]. Available from URL: http://company.merckserono.net [Accessed 2007 Sep 12]
Serono International SA. Serono submits new formulation of Rebif® for approval in the United States and in Europe (media release), 2006 Apr 4 [online]. Available from URL: http://company.merckserono.net [Accessed 2007 Sep 12]
De Stefano N, Beelke M. A randomised, placebo-controlled, double-blind, multicentre, phase IIIb study assessing efficacy, safety and tolerability of Rebif® new formulation in patients with relapsing-remitting multiple sclerosis [abstract plus poster]. 17th Annual Meeting of the European Neurological Society; 2007 Jun 16–20; Rhodes
Kappos L, Freedman M, De Stefano N, et al. REFLEX: a phase III trial to assess the effect of dosing frequency of interferon beta-1a (Rebif®) in delaying conversion of patients with a clinically isolated syndrome to clinically definite multiple sclerosis [abstract no. 409 plus poster]. 17th Annual Meeting of the European Neurological Society; 2007 Jun 16–20; Rhodes
Acknowledgements
The manuscript was reviewed by: B. M. Keegan, Department of Neurology, Mayo Clinic College of Medicine, Rochester, MN, USA; H. Panitch, University of Vermont College of Medicine, Burlington, VT, USA. During the peer review process, the manufacturer of the agent under review was also offered an opportunity to comment on this article. Changes based on any comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McKeage, K., Wagstaff, A.J. Subcutaneous Interferon-β-1a. CNS Drugs 21, 871–876 (2007). https://doi.org/10.2165/00023210-200721100-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00023210-200721100-00006