Abstract
Schizophrenia remains a severe disorder that is associated with a poor outcome in a large subgroup of patients. Major efforts should be made to improve treatment for all patients who have this debilitating disease. Second-generation antipsychotics were a major step forward in this respect; however, important unmet needs remain, such as a better solution for frequent noncompliance problems. Depot formulations are known to have advantages in this respect. However, for a long time, only depot formulations of conventional antipsychotics were available, with their high risk of extrapyramidal adverse effects. Therefore, there has been only very restricted use of depot antipsychotics, which mainly focused on patients with chronic disease who were difficult to treat and had a high risk of noncompliance. The situation may change with the advent of a depot formulation of an atypical antipsychotic.
The first depot formulation of an atypical antipsychotic to be introduced to the market is long-acting injectable risperidone. On the basis of the pharmacokinetic properties of the depot formulation, a 2-week interval between administrations is recommended. The antipsychotic efficacy of long-acting risperidone was demonstrated in two 12-week, double-blind, randomised, phase III studies, one versus placebo and the other versus oral risperidone. These two studies, together with one open-label, long-term study over 12 months, belong to the core group of trials that were relevant for the licensing of long-acting risperidone. A relapse-prevention, control group study comparing the long-acting formulation versus oral risperidone was not performed because of the known principal methodological problems of such a comparison. Instead, as much clinical data as possible was collected from observational studies that investigated questions relevant for clinical practice, such as efficacy, safety and tolerability in different subgroups, and transition from pre-treatment with different kinds of antipsychotics to long-acting risperidone. On the basis of these data, it can be stated that the efficacy of the long-term formulation of risperidone is proven, and that the safety and tolerability are more or less comparable to those of oral risperidone. The local tolerability at the injection site is good.
Because it is a well known fact that noncompliance is a frequent feature of the treatment of schizophrenia, and considering the advantages of atypical antipsychotics, consideration of whether long-acting atypical antipsychotics should have a broader indication than is the case with the depot formulations of the classical antipsychotics is warranted.
Similar content being viewed by others
References
Möller HJ, Bartender R, Groβ A, et al. The Kraepelinian dichotomy: preliminary results of a 15-year follow-up study on functional psychoses: focus on negative symptoms. Schizophr Res 2002; 56: 87–94
Möller HJ. Course and long-term treatment of schizophrenic psychoses. Pharmacopsychiatry 2004; 37 Suppl. 2: 126–35
Möller HJ. Definition, psychopharmacological basis and clinical evaluation of novel/atypical neuroleptics: methodological issues and clinical consequences. World J Biol Psychiatry 2000; 1: 75–91
Möller HJ. Antipsychotic agents. Gradually improving treatment from the traditional oral neuroleptics to the first atypical depot. Eur Psychiatry 2005; 20: 379–85
Weiden PJ, Olfson M. Cost of relapse in schizophrenia. Schizophr Bull 1995; 21: 419–29
Leucht S, Barnes T, Kissling W, et al. Relapse prevention in schizophrenia with new antipsychotics: a meta-analysis of randomized controlled trials. Am J Psychiatry 2003; 160: 1209–22
Bhanji NH, Chouinard G, Margolese HC. A review of compliance, depot intramuscular antipsychotics and the new long-acting injectable atypical antipsychotic risperidone in schizophrenia. Eur Neuropsychopharmacol 2004; 14: 87–92
Dolder CR, Lacro JP, Dunn LB, et al. Antipsychotic medication adherence: is there a difference between typical and atypical agents? Am J Psychiatry 2002; 159: 103–8
Möller HJ. Neuroleptische Langzeittherapie schizophrener Erkrankungen. In: Heinrich K, editor. Leitlinien neuroleptischer Therapie. Berlin: Springer, 1990: 97–115
Davis JM, Matalon L, Watanabe MD, et al. Depot antipsychotic drugs. Place in therapy. Drugs 1994; 47: 741–73
Adams CE, Fenton MK, Quraishi S, et al. Systematic meta-review of depot antipsychotic drugs for people with schizophrenia. Br J Psychiatry 2001; 179: 290–9
Kane JM. Dosing issues and depot medication in the maintenance treatment of schizophrenia. Int Clin Psychopharmacol 1995; 10 Suppl. 3: 65–71
Patel MX, Nikolaou V, David AS. Psychiatrists’ attitudes to maintenance medication for patients with schizophrenia. Psychol Med 2003; 33: 83–9
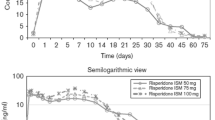
Ereshefsky L, Mannaert E. Pharmacokinetic profile and clinical efficacy of long-acting risperidone: potential benefits of combining an atypical antipsychotic and a new delivery system. Drugs R D 2005; 6: 129–37
Möller HJ, Kissling W. Advantages and disadvantages of depot-neuroleptics as maintenance medication for chronic schizophrenics. Clin Neuropharmacol 1986; 9 Suppl. 4: 259–62
Gerlach J. Depot neuroleptics in relapse prevention: advantages and disadvantages. Int Clin Psychopharmacol 1995; 9 Suppl. 5: 17–20
Midha KK, Hubbard JW, Marder SR, et al. Impact of clinical pharmacokinetics on neuroleptic therapy in patients with schizophrenia. J Psychiatry Neurosci 1994; 19: 254–64
Glazer WM. Review of incidence studies of tardive dyskinesia associated with typical antipsychotics. J Clin Psychiatry 2000; 61 Suppl. 4: 15–20
Chouinard G, Annable L, Ross-Chouinard A, et al. Factors related to tardive dyskinesia. Am J Psychiatry 1979; 136: 79–82
Llorca PM, Chereau I, Bayle FJ, et al. Tardive dyskinesias and antipsychotics: a review. Eur Psychiatry 2002; 17: 129–38
Chouinard G. Effects of risperidone in tardive dyskinesia: an analysis of the Canadian multicenter risperidone study. J Clin Psychopharmacol 1995; 15: 36–44S
Gerlach J. Improving outcome in schizophrenia: the potential importance of EPS and neuroleptic dysphoria. Ann Clin Psychiatry 2002; 14: 47–57
Correll CU, Leucht S, Kane JM. Lower risk for tardive dyskinesia associated with second-generation antipsychotics: a systematic review of 1-year studies. Am J Psychiatry 2004; 161: 414–25
Möller HJ. Risperidone: a review. Expert Opin Pharmacother 2005; 6: 803–18
Hamner M. The effects of atypical antipsychotics on serum prolactin levels. Ann Clin Psychiatry 2002; 14: 163–73
Csernansky JG, Mahmoud R, Brenner R. A comparison of risperidone and haloperidol for the prevention of relapse in patients with schizophrenia. N Engl J Med 2002; 346: 16–22
Ramstack M, Grandolfi GP, Mannaert E, et al. Long-acting risperidone: Prolonged-release injectable delivery of risperidone using medisorb microsphere technology. Schizophr Res 2003; 60 Suppl. 1: 87
Eerdekens M, Van Hove I, Remmerie B, et al. Pharmacokinetics and tolerability of long-acting risperidone in schizophrenia. Schizophr Res 2004; 70: 91–100
Nordstrom AL, Farde L, Wiesel FA, et al. Central D2-dopamine receptor occupancy in relation to antipsychotic drug effects: a double-blind PET study of schizophrenic patients. Biol Psychiatry 1993; 33: 227–35
Ereshefsky L, Lacombe S. Pharmacological profile of risperidone. Can J Psychiatry 1993; 38 Suppl. 3: S80–8
Gefvert O, Eriksson B, Persson P, et al. Pharmacokinetics and D2 receptor occupancy of long-acting injectable risperidone (Risperdal Consta) in patients with schizophrenia. Int J Neuropsychopharmacol 2005; 8: 27–36
Wilson WH. A visual guide to expected blood levels of long-acting injectable risperidone in clinical practice. J Psychiatr Pract 2004; 10: 393–401
Möller HJ, Llorca PM, Sacchetti E, et al. Efficacy and safety of direct transition to risperidone long-acting injectable in patients treated with various antipsychotic therapies. Int Clin Psychopharmacol 2005; 20: 121–30
Parellada E, Andrezina R, Milanova V, et al. Patients in the early phases of schizophrenia and schizoaffective disorders effectively treated with risperidone long-acting injectable. J Psychopharmacol 2005; 19: 5–14
Mohl A, Westlye K, Opjordsmoen S, et al. Long-acting risperidone in stable patients with schizoaffective disorder. J Psychopharmacol 2005; 19: 22–31
Kissling W, Heres S, Lloyd K, et al. Direct transition to long-acting risperidone: analysis of long-term efficacy. J Psychopharmacol 2005; 19: 15–21
Rubio G, Martinez I, Ponce G, et al. Long-acting injectable risperidone compared with zuclopenthixol in the treatment of schizophrenia with substance abuse comorbidity. Can J Psychiatry 2006; 51: 531–9
Gastpar M, Masiak M, Latif MA, et al. Sustained improvement of clinical outcome with risperidone long-acting injectable in psychotic patients previously treated with olanzapine. J Psychopharmacol 2005; 19: 32–8
Turner M, Eerdekens E, Jacko M, et al. Long-acting injectable risperidone: safety and efficacy in stable patients switched from conventional depot antipsychotics. Int Clin Psychopharmacol 2004; 19: 241–9
Chue P, Eerdekens M, Augustyns I, et al. Comparative efficacy and safety of long-acting risperidone and risperidone oral tablets. Eur Neuropsychopharmacol 2005; 15: 111–7
Lasser RA, Bossie CA, Gharabawi GM, et al. Remission in schizophrenia: results from a 1-year study of long-acting risperidone injection. Schizophr Res 2005; 77: 215–27
Fleischhacker WW, Eerdekens M, Karcher K, et al. Treatment of schizophrenia with long-acting injectable risperidone: a 12-month open-label trial of the first long-acting second-generation antipsychotic. J Clin Psychiatry 2003; 64: 1250–7
Gharabawi GM, Lasser RA, Bossie CA, et al. Insight and its relationship to clinical outcomes in patients with schizophrenia or schizoaffective disorder receiving long-acting risperidone. Int Clin Psychopharmacol 2006; 21: 233–40
Chue P, Llorca PM, Duchesne I, et al. Hospitalization rates in patients during long-term treatment with long-acting risperidone injection. J of Applied Research 2005; 5: 266–74
Fleischhacker WW, Rabinowitz J, Kemmler G, et al. Perceived functioning, well-being and psychiatric symptoms in patients with stable schizophrenia treated with long-acting risperidone for 1 year. Br J Psychiatry 2005; 187: 131–6
Gharabawi GM, Bossie CA, Zhu Y, et al. An assessment of emergent tardive dyskinesia and existing dyskinesia in patients receiving long-acting, injectable risperidone: results from a long-term study. Schizophr Res 2005; 77: 129–39
Lindenmayer JP, Jarboe K, Bossie CA, et al. Minimal injection site pain and high patient satisfaction during treatment with long-acting risperidone. Int Clin Psychopharmacol 2005; 20: 213–21
Lasser RA, Bossie CA, Gharabawi GM, et al. Clinical improvement in 336 stable chronically psychotic patients changed from oral to long-acting risperidone: a 12-month open trial. Int J Neuropsychopharmacol 2005; 8: 427–38
Lasser R, Bossie CA, Gharabawi G, et al. Efficacy and safety of long-acting risperidone in stable patients with schizoaffective disorder. J Affect Disord 2004; 83: 263–75
Leal A, Rosillon D, Mehnert A, et al. Healthcare resource utilization during 1-year treatment with long-acting, injectable risperidone. Pharmacoepidemiol Drug Saf 2004; 13: 811–6
Lasser RA, Bossie CA, Zhu Y, et al. Efficacy and safety of long-acting risperidone in elderly patients with schizophrenia and schizoaffective disorder. Int J Geriatr Psychiatry 2004; 19: 898–905
van Os J, Bossie CA, Lasser RA. Improvements in stable patients with psychotic disorders switched from oral conventional antipsychotics therapy to long-acting risperidone. Int Clin Psychopharmacol 2004; 19: 229–32
Lasser RA, Bossie CA, Gharabawi GM, et al. Patients with schizophrenia previously stabilized on conventional depot antipsychotics experience significant clinical improvements following treatment with long-acting risperidone. Eur Psychiatry 2004; 19: 219–25
Kane JM, Eerdekens M, Lindenmayer JP, et al. Long-acting injectable risperidone: efficacy and safety of the first long-acting atypical antipsychotic. Am J Psychiatry 2003; 160: 1125–32
Ciliberto N, Bossie CA, Urioste R, et al. Lack of impact of race on the efficacy and safety of long-acting risperidone versus placebo in patients with schizophrenia or schizoaffective disorder. Int Clin Psychopharmacol 2005; 20: 207–12
Lauriello J, McEvoy JP, Rodriguez S, et al. Long-acting risperidone vs. placebo in the treatment of hospital inpatients with schizophrenia. Schizophr Res 2005; 72: 249–58
Nasrallah HA, Duchesne I, Mehnert A, et al. Health-related quality of life in patients with schizophrenia during treatment with long-cfu injectable risperidone. J Clin Psychiatry 2004; 65: 531–6
Lindenmayer JP, Eerdekens E, Berry SA, et al. Safety and efficacy of long-acting risperidone in schizophrenia: a 12-week, multicenter, open-label study in stable patients switched from typical and atypical oral antipsychotics. J Clin Psychiatry 2004; 65: 1084–9
Simpson GM, Mahmoud RA, Lasser RA, et al. A 1-year double-blind study of 2 doses of long-acting risperidone in stable patients with schizophrenia or schizoaffective disorder. J Clin Psychiatry 2006; 67: 1194–203
Bai YM, Chen TT, Wu B, et al. A comparative efficacy and safety study of long-acting risperidone injection and risperidone oral tablets among hospitalized patients: 12-week randomized, single-blind study. Pharmacopsychiatry 2006; 39: 135–41
Taylor DM, Young C, Patel MX. Prospective 6-month follow-up of patients prescribed risperidone long-acting injection: factors predicting favourable outcome. Int J Neuropsychopharmacol 2006; 9: 685–94
Young CL, Taylor DM. Health resource utilization associated with switching to risperidone long-acting injection. Acta Psychiatr Scand 2006; 114: 14–20
Chouinard G, Ross-Chouinard A, Annable L, et al. Extrapyramidal Symptom Rating Scale. Can J Neurol Sci 1980; 7: 233
Andreasen NC, Carpenter WT Jr, Kane JM, et al. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry 2005; 162: 441–9
Taylor DM, Young CL, Mace S, et al. Early clinical experience with risperidone long-acting injection: a prospective, 6-month follow-up of 100 patients. J Clin Psychiatry 2004; 65: 1076–83
Patel MX, Young C, Samele C, et al. Prognostic indicators for early discontinuation of risperidone long-acting injection. Int Clin Psychopharmacol 2004; 19: 233–9
Lambert M, Naber D. Current issues in schizophrenia: overview of patient acceptability, functioning capacity and quality of life. CNS Drugs 2004; 18 Suppl. 2: 5–17; discussion 43
Möller HJ. State of the art of drug treatment of schizophrenia and the future position of the novel/atypical antipsychotics. World J Biol Psychiatry 2000; 1: 204–14
Falkai P, Wobrock T, Lieberman J, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, Part 1: acute treatment of schizophrenia. World J Biol Psychiatry 2005; 6: 132–91
Falkai P, Wobrock T, Lieberman J, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, Part 2: long-term treatment of schizophrenia. World J Biol Psychiatry 2006; 7: 5–40
Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999; 156: 1686–96
Anonymous. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 2004; 27: 596–601
Möller HJ. Long-acting risperidone-focus on safety. Clin Ther 2006; 28: 633–51
Scandinavian Society of Psychopharmacology Committee of Clinical Investigations. The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: combined results of the North American trials. Acta Psychiatr Scand Suppl 1987; 334: 81–94
Love RC, Nelson MW. Pharmacology and clinical experience with risperidone. Expert Opin Pharmacother 2000; 1: 1441–53
Liu GG, Sun SX, Christensen DB, et al. Cost comparisons of olanzapine and risperidone in treating schizophrenia. Ann Pharmacother 2004; 38: 134–41
Shaw JW. Economic evaluations of olanzapine and risperidone. Am J Health Syst Pharm 2002; 59: 1366–75
Revicki DA. Pharmacoeconomic studies of atypical antipsychotic drugs for the treatment of schizophrenia. Schizophr Res 1999; 35 Suppl.: S101–9
Zito JM. Pharmacoeconomics of the new antipsychotics for the treatment of schizophrenia. Psychiatr Clin North Am 1998; 21: 181–202
Davies A, Langley PC, Keks NA, et al. Risperidone versus haloperidol: II. Cost-effectiveness. Clin Ther 1998; 20: 196–213
Foster RH, Goa KL. Risperidone. A pharmacoeconomic review of its use in schizophrenia. Pharmacoeconomics 1998; 14: 97–133
Remington G, Devos E, Eerdekens M, et al. Treatment with long-acting risperidone injection reduces healthcare resource use. Int J Neuropsychopharmacol 2002; 5 Suppl. 1: S189
Chue PS, Devos E, Duchesne IY, et al. One year hospitalization rates in patients with schizophrenia during long-term treatment with long-acting risperidone. Value Health 2002; 5: 229
Llorca PM, Devos E, Eerdekens M, et al. Re-hospitalization rates with long-acting risperidone injection are lower than those reported for other antipsychotics. Int J Neuropsychopharmacol 2002; 5 Suppl. 1: S189
Eriksson L, Almqvist A, Mehnert A, et al. Long-acting risperidone significantly reduces the need for institutional psychiatric care. Poster at the American College of Neuropsychopharmacology 42nd Annual Meeting; 7–11 Dec 2003, San Juan
Llorca PM, Jasso-Mosqueda JG, Miadi-Fargier H, et al. Long-acting risperidone injection in patients with schizophrenia compared with oral olanzapine and haloperidol decanoate: results of a cost-effectiveness model. Poster presented at the 16th European College of Neuropsychopharmacology Congress; 2003 Sep 20–24; Prague
Rabinowitz J, Lichtenberg P, Kaplan Z, et al. Rehospitalization rates of chronically ill schizophrenic patients discharged on a regimen of risperidone, olanzapine, or conventional antipsychotics. Am J Psychiatry 2001; 158: 266–9
Heeg BM, van Aalst G, van den Arend IJ, et al. A discrete events model of long-term outcomes and cost of treatment with long-acting risperidone in schizophrenia. Value Health 2002; 5: 515–6
Heeg BM, Emmermann A, Laux G, et al. Costs and effects of Risperdal Consta™ in comparison to conventional depot and short-acting atypical formulations in Germany. Value Health 2003; 6: 692
Harrison TS, Goa KL. Long-acting risperidone: a review of its use in schizophrenia. CNS Drugs 2004; 18: 113–32
Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005; 353: 1209–23
Coldham EL, Addington J, Addington D. Medication adherence of individuals with a first episode of psychosis. Acta Psychiatr Scand 2002; 106: 286–90
Kamali M, Kelly BD, Clarke M, et al. A prospective evaluation of adherence to medication in first episode schizophrenia. Eur Psychiatry 2006; 21: 29–33
Wobrock T, Sittinger H, Behrendt B, et al. Comorbid substance abuse and neurocognitive function in recent-onset schizophrenia. Eur Arch Psychiatry Clin Neurosci 2006. Epub ahead of print
Marder SR, Conley R, Ereshefsky L, et al. Clinical guidelines: dosing and switching strategies for long-acting risperidone. J Clin Psychiatry 2003; 64 Suppl. 16: 41–6
Keith SJ, Pani L, Nick B, et al. Practical application of pharmacotherapy with long-acting risperidone for patients with schizophrenia. Psychiatr Serv 2004; 55: 997–1005
Masand PS, Gupta S. Long-acting injectable antipsychotics in the elderly: guidelines for effective use. Drugs Aging 2003; 20: 1099–110
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Möller, HJ. Long-Acting Injectable Risperidone for the Treatment of Schizophrenia. Drugs 67, 1541–1566 (2007). https://doi.org/10.2165/00003495-200767110-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200767110-00003