Summary
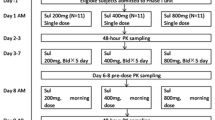
The pharmacokinetics of oral cibenzoline were studied in 30 arrhythmia patients as part of an ascending multiple-dose efficacy study. The elimination half-life of the drug following repetitive dosing ranged from 7.6 to 22.3 hours, with a harmonic mean of 12.3 hours (n = 24), and increased with age and decreasing renal function. The drug exhibited apparent dose proportional and linear pharmacokinetics over the range of doses studied. Multivariate analysis revealed that the patients’ age and serum creatinine concentration accounted for 71% of the variability in the range of β values (terminal elimination rate constant), and that 69.5% of the intersubject variability in the steady-state trough plasma concentrations could be accounted for by the patients’ age, weight and serum creatinine concentration.
These data suggest that, although there is some intersubject variability in the elimination and accumulation of cibenzoline, much of the variability can be explained by the patients’ age, weight and renal function.
Similar content being viewed by others
References
Brazzell, R.K.; Aogaichi, K.; Heger, J.J.; Somberg, J.C.; Carliner, N.H. and Morganroth, J.: Relationship between cibenzoline plasma concentration and antiarrhythmic effect. Clinical Pharmacology and Therapeutics 35: 307–316 (1984).
Canal, M.; Flouvat, B.; Tremblay, D. and Dufour, A.: Pharmacokinetics in man of a new antiarrhythmic drug, cibenzoline. European Journal of Clinical Pharmacology 24: 509–515 (1983).
Cockcroft, D.W. and Gault, M.H.: Prediction of creatinine clearance from serum creatinine. Nephron 16: 31–41 (1976).
Gibaldi, M. and Perrier, D. (Eds): Pharmacokinetics. p. 124 (Marcel Dekker Inc, New York 1975).
Hackman, M.R.; Lee, T.L. and Brooks, M.A.: Determination of cibenzoline in plasma and urine by high pressure liquid chromatography. Journal of Chromatography 273: 347–356 (1983).
Metzler, C.M.; Elfring, G.L. and McEwen, A.J.: Package of computer programs for pharmacokinetic modeling. Biometrics 30: 562–563 (1974).
Millar, J.S. and Vaughan Williams, E.M.: Effects on rabbit nodal, atrial, ventricular and Purkinje cell potentials of a new antiarrhythmic drug, cibenzoline, which protects against action potential shortening in hypoxia. British Journal of Pharmacology 75: 469–478 (1982).
Tepper, D.; Butler, B.; Keren, G.; Somberg, J.C.; Taragin, M.; Siegel, L.; Aogaichi, K. and Miura, D.S.: Effects of oral cibenzoline therapy on ventricular ectopic activity. Clinical Research 31(2): A634 (1983).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brazzell, R.K., Colburn, W.A., Aogaichi, K. et al. Pharmacokinetics of Oral Cibenzoline in Arrhythmia Patients. Clin Pharmacokinet 10, 178–186 (1985). https://doi.org/10.2165/00003088-198510020-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-198510020-00005