Abstract
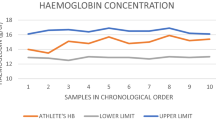
The stability of haematological parameters is crucial to guarantee accurate and reliable data for implementing and interpreting the athlete’s biological passport (ABP). In this model, the values of haemoglobin, reticulocytes and out-of-doping period (OFF)-score (Hb-60√Ret) are used to monitor the possible variations of those parameters, and also to compare the thresholds developed by the statistical model for the single athlete on the basis of its personal values and the variance of parameters in the modal group. Nevertheless, a critical review of the current scientific literature dealing with the stability of the haematological parameters included in the ABP programme, and which are used for evaluating the probability of anomalies in the athlete’s profile, is currently lacking. In addition, we collected information from published studies, in order to supply a useful, practical and updated review to sports physicians and haematologists.
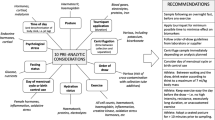
There are some parameters that are highly stable, such as haemoglobin and erythrocytes (red blood cells [RBCs]), whereas others, (e.g. reticulocytes, mean RBC volume and haematocrit) appear less stable. Regardless of the methodology, the stability of haematological parameters is improved by sample refrigeration. The stability of all parameters is highly affected from high storage temperatures, whereas the stability of RBCs and haematocrit is affected by initial freezing followed by refrigeration. Transport and rotation of tubes do not substantially influence any haematological parameter except for reticulocytes.
In all the studies we reviewed that used Sysmex instrumentation, which is recommended for ABP measurements, stability was shown for 72 hours at 4°C for haemoglobin, RBCs and mean curpuscular haemoglobin concentration (MCHC); up to 48 hours for reticulocytes; and up to 24 hours for haematocrit. In one study, Sysmex instrumentation shows stability extended up to 72 hours at 4°C for all the parameters. There are significant differences among methods and instruments: Siemens Advia shows lower stability than Sysmex as regards to reticulocytes. However, the limit of 36 hours from blood collection to analysis as recommended by ABP scientists is reasonable to guarantee analytical quality, when samples are transported at 4°C and are accompanied by a certified steadiness of this temperature.
There are some parameters that are highly stable, such as haemoglobin and RBCs; whereas others, such as reticulocytes, mean cell volume and haematocrit are more unstable. The stability of haematological parameters might be improved independently from the analytical methodology, by refrigeration of the specimens.




Similar content being viewed by others
References
Sottas PE, Robinson N, Saugy M. The athlete’s biological passport and indirect markers of blood doping. Handb Exp Pharmacol 2010 (195): 305–26
Banfi G, Dolci A. Preanalytical phase of sport biochemistry and haematology. J Sports Med Phys Fitness 2003; 43 (2): 223–30
Parisotto R, Gore CJ, Emslie KR, et al. A novel method utilising markers of altered erythropoiesis for the detection of recombinant human erythropoietin abuse in athletes. Haematologica 2000; 85 (6): 564–72
Banfi G. Reticulocytes in sports medicine. Sports Med 2008; 38 (3): 187–211
Sharpe K, Ashenden MJ, Schumacher YO. A third generation approach to detect erythropoietin abuse in athletes. Haematologica 2006; 91: 356–63
Berg B, Estborn B, Tryding N. Stability of serum and blood constituents during mail transport. Scand J Clin Lab Invest 1981; 41 (5): 425–30
Felding P, Petersen PH, Horder M. The stability of blood, plasma and serum constituents during simulated transport. Scand J Clin Lab Invest 1981; 41 (1): 35–40
WHO. Use of anticoagulants in diagnostic laboratory investigations & stability of blood, plasma and serum samples 2002; WHO/DIL/LAB/99.1 Rev. 2. Contributors: Banfi G, Bauer K, Brand W, et al. [online]. Available from URL: http://whqlibdoc.who.int/hq/2002/WHO_DIL_LAB_99.1_Rev.2.pdf [Accessed 2011 Sep 26]
Imeri F, Herklotz R, Risch L, et al. Stability of hematological analytes depends on the hematology analyser used: a stability study with Bayer Advia 120, Beckman Coulter LH 750 and Sysmex XE 2100. Clin Chim Acta 2008; 397: 68–71
Robinson N, Mangin P, Saugy M. Time and temperature dependant changes in red blood cell analytes used for testing recombinant erythropoietin abuse in sports. Clin Lab 2004; 50 (5-6): 317–23
Robinson N, Sottas PE, Pottgiesser T, et al. Stability and robustness of blood variables in an antidoping context. Int J Lab Hematol 2011; 33: 146–53
Voss SC, Flenker U, Majer B, et al. Stability tests for hematological parameters in antidoping analyses. Lab Hematol 2008; 14: 24–9
Kouri T, Siloaho M, Pohjavaara S, et al. Pre-analytical factors and measurement uncertainty. Scand J Clin Lab Invest 2005; 65: 463–75
Lippi G, Salvagno GL, Solero GP, et al. Stability of blood cell counts, hematologic parameters and reticulocytes indexes on the Advia A120 hematologic analyzer. J Lab Clin Med 2005; 146: 333–40
Sottas PE, Robinson N, Saugy M, et al. A forensic approach to the interpretation of blood doping markers. Law Prob Risk 2008; 7: 191–210
Bourner G, Dhaliwal J, Sumner J. Performance evaluation of the latest fully automated hematology analyzers in a large, commercial laboratory setting: a 4-way, side-by-side study. Lab Hematol 2005; 11 (4): 285–97
Wiegand G, Effenberger-Klein A, Weber R, et al. Potential pitfalls of comparative measurements of reticulocytes with flow cytometry and microscopy in prematures and infants. Clin Chem Lab Med 2004; 42 (10): 1150–4
Banfi G. Reticulocytes in sports medicine. Sports Med 2008; 38: 187–211
Cavill I, Kraaijenhagen R, Pradella R, et al. In vitro stability of the reticulocyte count. Clin Lab Haematol 1996; 18 Suppl.1: 9–11
Hamilton PJ, Davidson RL. The interrelationship and stability of Coulter S-determined blood indices. J Clin Path 1973; 30: 54–7
Johnson M, Samuels C, Samuels C, et al. Three-way evaluation of high throughput haematology analysers: Beckman Coulter LH 750, Abbott Cell-Dyn 4000, and Sysmex XE 2100. Lab Hematol 2002; 8: 230–8
Lippi G, Banfi G, Maffulli N. Preanalytical variability: the dark side of the moon in blood doping screening. Eur J Appl Physiol 2010; 109 (5): 1003–5
Thiers RE, Wu GT, Reed AH, et al. Sample stability: a suggested definition and method of determination. Clin Chem 1976; 22 (2): 176–83
Acknowledgements
The authors have no conflicts of interest that are directly relevant to the content of this review. No funds were used to assist in the preparation of this review.
G. Banfi gave expert testimony for two athletes at the Court of Arbitration for Sport hearings regarding ABP, but this is not relevant to the present review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lombardi, G., Lanteri, P., Colombini, A. et al. Stability of Haematological Parameters and Its Relevance on the Athlete’s Biological Passport Model. Sports Med 41, 1033–1042 (2011). https://doi.org/10.2165/11591460-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11591460-000000000-00000