Abstract
Background and Objectives: Rivastigmine is approved for the symptomatic treatment of mild to moderate dementia in patients with Alzheimer’s disease. The drug was launched in Taiwan in 2000. The primary objective of this post-marketing surveillance (PMS) study was to describe the safety/tolerability of treatment with rivastigmine capsules in patients with Alzheimer’s disease. The secondary objectives of this study were to define the optimal titration pattern, maintenance dose, efficacy and patient satisfaction with treatment with rivastigmine capsules.
Methods: This was a prospective, non-interventional post-marketing observational study in patients who met the criteria for mild or moderate Alzheimer’s disease. The primary outcome measure for this trial was the incidence of emerging adverse events. Dosages related to titration patterns and maintenance doses were summarized. Efficacy evaluations conducted using the Mini-Mental State Examination, Clinical Dementia Rating and modified Instrumental Activities of Daily Living scales were also primary outcome measures, and results are shown descriptively. The patients’ therapeutic responses to rivastigmine and satisfaction with rivastigmine were secondary outcome measures. Therapeutic response and treatment satisfaction were summarized descriptively.
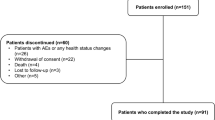
Results: A total of 264 patients were enrolled into the study. The mean duration of exposure to rivastigmine during the study was 151.1 days. Patients were taking rivastigmine 1.5–6 mg twice daily and the most frequent maintenance dose level was 4.5 mg twice daily. Among patients treated with rivastigmine, all primary and secondary outcome measures showed improvement or stabilization of cognition and global functioning. Of the 253 safety analysis patients, 155 patients (61.3%) reported at least one adverse event. The most frequent adverse events by system organ class were psychiatric disorders (9.1%) and gastrointestinal disorders (8.3%). The most common adverse events observed were dizziness (5.5%), insomnia (5.1%), anorexia (4.0%) and gastrointestinal symptoms such as constipation (4.0%), vomiting (4.0%) and nausea (3.6%). These symptoms were mild in severity. A total of 12 patients (4.7%) reported 16 serious adverse events, including two deaths, three fractures, three behavioural and psychological symptoms of dementia, one syncope with head trauma, one peptic ulcer, and six other hospitalizations. None were reported to be related to rivastigmine.
Conclusions: Based on the results of this study, rivastigmine administered usually at a dose of 3–6 mg twice daily was found to be well tolerated. Although the rate of adverse events was high, the majority of these symptoms were mild in severity and short in duration. This study also demonstrated the efficacy of rivastigmine in at least stabilizing the symptoms of Alzheimer’s disease.






Similar content being viewed by others
References
Graves AB, Kukull WA. The epidemiology of dementia. In: Morris JC, editor. Handbook of dementing illnesses. New York: Marcel Dekker, 1994: 23–69
Weinstock M. Selectivity of cholinesterase inhibition. CNS Drugs 1999; 12: 307–23
Ballard CG. Advances in the treatment of Alzheimer’s disease: benefits of dual cholinesterase inhibition. Eur Neurol 2002; 47: 64–70
Corey-Bloom J, Anand R, Veach J. A randomized trial evaluating the efficacy and safety of ENA 713 (rivastigmine tartrate), a new acetylcholinesterase inhibitor, in patients with mild to moderately severe Alzheimer’s disease. Int J Geriatr Psychopharmacol 1998; 1: 55–65
Rösler M, Anand R, Cicin-Sain A, et al. Efficacy and safety of rivastigmine in patients with Alzheimer’s disease: international randomized controlled trial. BMJ 1999; 318: 633–40
Small GW, Kaufer D, Mendiondo MS, et al. Cognitive performance in Alzheimer’s disease patients receiving rivastigmine for up to 5 years. Int J Clin Pract 2005; 59: 473–7
Plosker GL, Keating GM. Management of mild to moderate Alzheimer disease: defining the role of rivastigmine. Dis Manage Health Outcomes 2004; 12: 55–72
Raina P, Santaguida P, Ismaila A, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med 2008; 148: 379–97
Grossberg G, Irwin P, Satlin A, et al. Rivastigmine in Alzheimer disease: efficacy over two years. Am J Geriatr Psychiatry 2004; 12: 420–31
Kavirajan H, Schneider LS. Efficacy and adverse effects of cholinesterase inhibitors and memantine in vascular dementia: a meta-analysis of randomized controlled trials. Lancet Neurol 2007; 6: 782–92
Hogan DB, Bailey P, Black S, et al. Diagnosis and treatment of dementia: 5. Nonpharmacologic and pharmacologic therapy for mild to moderate dementia. CMAJ 2008; 179: 1019–26
Sikdar S. Should titration schedules for cholinesterase inhibitors be changed? Int J Geriatr Psychiatry 2003; 18: 1063–4
Farlow M, Anand R, Messina Jr J, et al. A 52-week study of the efficacy of rivastigmine in patients with mild to moderately severe Alzheimer’s disease. Eur Neurol 2000; 44: 234–41
Potkin SG, Anand R, Hartman R, et al. Impact of Alzheimer’s disease and rivastigmine treatment on activities of daily living over the course of mild to moderately severe disease. Prog Neuropsychopharmacol Biol Psychiatry 2002; 26: 713–20
Aguglia E, Onor ML, Saina M, et al. An open-label, comparative study of rivastigmine, donepezil and galantamine in a real-world setting. Curr Med Res Opin 2004; 20: 1747–52
Holmes C, Lovestone S. Long-term cognitive and functional decline in late onset Alzheimer’s disease: therapeutic implications. Age Ageing 2003; 32: 200–4
Dooley M, Lamb HM. Donepezil: a review of its use in Alzheimer’s disease. Drugs Aging 2000; 16: 199–226
Acknowledgements
This multicentre PMS study was conducted without any funding except for assistance with the completion of case report forms and statistical analysis by Novartis. The authors would like to thank Novartis Pharma AG, Basel, Switzerland, for this support. In addition, we wish to express our appreciation to other participants who provided valuable assistance with regard to this study including: Dr Liang-Huey Chang, Dr Yiao-Cheung Cheng, Dr Chi-Hsiang Chou, Dr Wei-Chih Hsu, Dr Chin-Chang Huang, Dr Jiunn-Hong Jou, Dr Hui-Ju Kao, Dr Yam-Ting Kwok, Dr Jiann-Der Lee, Dr Jun-Cheng Lin, Dr Ming-Hui Sun, Dr Hsiang-Chang Wang and Dr Shang-Te Wu. The authors have no conflicts of interest that are directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chiu, PY., Dai, DE., Hsu, HP. et al. Safety/Tolerability and Efficacy of Rivastigmine in Taiwanese Patients with Alzheimer’s Disease. Clin. Drug Investig. 29, 729–738 (2009). https://doi.org/10.2165/11315320-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11315320-000000000-00000