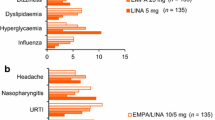
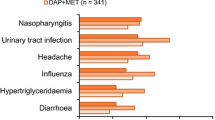
Abstract
Linagliptin (Trajenta®, Tradjenta™, Trazenta™, Trayenta™) is an oral, highly selective inhibitor of dipeptidyl peptidase-4 and is the first agent of its class to be eliminated predominantly via a nonrenal route. Linagliptin is indicated for once-daily use for the treatment of adults with type 2 diabetes mellitus, and a twice-daily fixed-dose combination of linagliptin/metformin (Jentadueto®) is lso available. In this article, the pharmacological, clinical efficacy and tolerability data relevant to the use of linagliptin in patients with type 2 diabetes are reviewed.
The efficacy of oral linagliptin in the treatment of adults with type 2 diabetes has been investigated in several double-blind, multicentre trials. Following 12–24 weeks of treatment, improvements in glycaemic control parameters, including glycosylated haemoglobin (HbA1c; primary endpoint in all trials), were seen with linagliptin relative to placebo when used as monotherapy, initial combination therapy (with metformin or pioglitazone) or add-on therapy to other oral anti-hyperglycaemia agents (metformin and/or a sulfonylurea) or basal insulin (with or without metformin and/or pioglitazone). In terms of lowering HbA1c, linagliptin was more effective than voglibose in a 26-week monotherapy trial and noninferior to glimepiride when used as add-on therapy to metformin in a 104-week study. Additional trials and subgroup analyses of pooled data suggest that linagliptin improves glycaemic control regardless of factors such as age, duration of type 2 diabetes, ethnicity and renal function, and as linagliptin is eliminated primarily via a nonrenal route, it can be used without dosage adjustment in patients with renal impairment of any degree. Oral linagliptin was generally well tolerated and was associated with a low likelihood of hypoglycaemia (except when used in combination with a sulfonylurea) and had little effect on bodyweight.
Further long-term and comparative efficacy and tolerability data are required to help position linagliptin more definitively with respect to other antihyperglycaemia agents. However, clinical data currently available indicate that linagliptin is an effective and generally well tolerated treatment option for use in patients with type 2 diabetes, including those with renal impairment for whom other antihyperglycaemia agents require dosage adjustment or are not suitable.









Similar content being viewed by others
References
Davidson JA. Advances in therapy for type 2 diabetes: GLP-1 receptor agonists and DPP-4 inhibitors. Clev Clin J Med 2009 Dec; 76 Suppl. 5: S28–38
Tahrani AA, Bailey CJ, Del Prato S, et al. Management of type 2 diabetes: new and future developments in treatment. Lancet 2011 Jul; 378 (9786): 182–97
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2008 Jan; 31 Suppl. 1: S62-7
National Institute for Health and Clinical Excellence. Type 2 diabetes: newer agents [online]. Available from URL: http://www.nice.org.uk/nicemedia/live/12165/44318/44318.pdf [Accessed 2012 May 9]
Nolan CJ, Damm P, Prentki M. Type 2 diabetes across generations: from pathophysiology to prevention and management. Lancet 2011 Jul 9; 378 (9786): 169–81
Drucker DJ. The role of gut hormones in glucose homeostasis. J Clin Invest 2007 Jan; 117 (1): 24–32
Holst JJ, McGill MA. Potential new approaches to modifying intestinal GLP-1 secretion in patients with type 2 diabetes mellitus: focus on bile acid sequestrants. Clin Drug Investig 2012 Jan 1; 32 (1): 1–14
Scott LJ. Linagliptin: in type 2 diabetes mellitus. Drugs 2011; 71 (5): 611–24
Del Prato S, Barnett AH, Huisman H, et al. Effect of linagliptin monotherapy on glycaemic control and markers of b-cell function in patients with inadequately controlled type 2 diabetes: a randomized controlled trial. Diabetes Obes Metab 2011 Mar; 13 (3): 258–67
Kawamori R, Inagaki N, Araki E, et al. Linagliptin monotherapy provides superior glycaemic control versus placebo or voglibose with comparable safety in Japanese patients with type 2 diabetes: a randomized, placebo and active comparator-controlled, double-blind study. Diabetes Obes Metab 2012 Apr; 14 (4): 348–57
Forst T, Uhlig-Laske B, Ring A, et al. Linagliptin (BI 1356), a potent and selective DPP-4 inhibitor, is safe and efficacious in combination with metformin in patients with inadequately controlled type 2 diabetes. Diabet Med 2010 Dec; 27 (12): 1409–19
Taskinen MR, Rosenstock J, Tamminen I, et al. Safety and efficacy of linagliptin as add-on therapy to metformin in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab 2011 Jan; 13 (1): 65–74
Gomis R, Espadero R-M, Jones R, et al. Efficacy and safety of initial combination therapy with linagliptin and pioglitazone in patients with inadequately controlled type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab 2011 Jul; 13 (7): 653–61
Owens DR, Swallow R, Dugi KA, et al. Efficacy and safety of linagliptin in persons with type 2 diabetes inadequately controlled by a combination of metformin and sulphonylurea: a 24-week randomized study [published erratum appears in Diabet Med 2012; 29 (1): 158]. Diabet Med 2011 Nov; 28 (11): 1352–61
Heise T, Graefe-Mody EU, Hüttner S, et al. Pharmacokinetics, pharmacodynamics and tolerability of multiple oral doses of linagliptin, a dipeptidyl peptidase-4 inhibitor in male type 2 diabetes patients. Diabetes Obes Metab 2009 Aug; 11 (8): 786–94
Horie Y, Kanada S, Watada H, et al. Pharmacokinetic, pharmacodynamic, and tolerability profiles of the dipeptidyl peptidase-4 inhibitor linagliptin: a 4-week multi-center, randomized, double-blind, placebo-controlled phase IIa study in Japanese type 2 diabetes patients. Clin Ther 2011 Jul; 33 (7): 973–89
Forst T, Uhlig-Laske B, Ring A, et al. The oral DPP-4 inhibitor linagliptin significantly lowers HbA1c after 4 weeks of treatment in patients with type 2 diabetes mellitus. Diabetes Obes Metab 2011 Jun; 13 (6): 542–50
Gallwitz B, Rosenstock J, Rauch T, et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. Lancet. Epub 2012 Jun 27
Sarashina A, Sesoko S, Nakashima M, et al. Linagliptin, a dipeptidyl peptidase-4 inhibitor in development for the treatment of type 2 diabetes mellitus: a phase I, randomized, double-blind, placebo-controlled trial of single and multiple escalating doses in healthy adult male Japanese subjects. Clin Ther 2010 Jun; 32 (6): 1188–204
Hüttner S, Graefe-Mody EU, Withopf B, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of single oral doses of BI 1356, an inhibitor of dipeptidyl peptidase 4, in healthy male volunteers. J Clin Pharmacol 2008 Oct; 48 (10): 1171–8
Retlich S, Duval V, Ring A, et al. Pharmacokinetics and pharmacodynamics of single rising intravenous doses (0.5 mg–10 mg) and determination of absolute bioavailability of the dipeptidyl peptidase-4 inhibitor linagliptin (BI 1356) in healthy male subjects. Clin Pharmacokinet 2010 Dec; 49 (12): 829–40
Singh-Franco D, McLaughlin-Middlekauff J, Elrod S, et al. The effect of linagliptin on glycaemic control and tolerability in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Obes Metab 2012 Aug; 14 (8): 694–708
Eckhardt M, Langkopf E, Mark M, et al. 8-(3-(R)-amino-piperidin-1-yl)-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazolin-2-ylmethyl)-3,7-dihydropurine-2,6-dione (BI 1356), a highly potent, selective, long-acting, and orally bioavailable DPP-4 inhibitor for the treatment of type 2 diabetes. J Med Chem 2007 Dec; 50 (26): 6450–3
Thomas L, Eckhardt M, Langkopf E, et al. (R)-8-(3-amino-piperidin-1-yl)-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazolin-2-ylmethyl)-3,7-dihydro-purine-2,6-dione (BI 1356), a novel xanthine-based dipeptidyl peptidase 4 inhibitor, has a superior potency and longer duration of action compared with other dipeptidyl peptidase-4 inhibitors. J Pharmacol Exp Ther 2008 Apr; 325 (1): 175–82
Schuff A, Steven S, Schell R, et al. Comparison of the direct and indirect antioxidant effects of DPP-4 inhibitors: the anti-inflammatory and vasodilatory potential of linagliptin [abstract no. 981-P]. Diabetes 2011; 60 Suppl. 1: A269
Sharkovska Y, Alter M, Reichetzeder C, et al. Renoprotective effects of the DPP-4 inhibitor linagliptin in db/db mice [abstract no. 986-P plus poster]. 72nd Scientific Sessions of the American Diabetes Association; 2012 Jun 8–12; Philadelphia (PA)
Klein T, Niessen HG, Ittrich C, et al. Evaluation of body fat composition after linagliptin treatment in a rat model of diet-induced obesity: a magnetic resonance spectroscopy study in comparison with sibutramine. Diabetes Obes Metab. Epub 2012 May 31
Chaykovska L, von Websky K, Rahnenführer J, et al. Effects of DPP-4 inhibitors on the heart in a rat model of uremic cardiomyopathy. PLoS One 2011 Nov; 6 (11): e27861
Vickers SP, Cheetham SC, Birmingham GD, et al. The DPP-4 inhibitor linagliptin is weight neutral in the DIO rat but inhibits the weight gain of DIO animals withdrawn from exenatide [abstract no. 979-P]. Diabetes 2011; 60 Suppl. 1: A268
Hocher B, Sharkovska Y, Mark M, et al. The novel DPP-4 inhibitors linagliptin and BI 14361 reduce infarct size after myocardial ischemia/reperfusion in rats. In J Cardiol. Epub 2012 Jan 2
Klein T, Batra A, Mark M, et al. The DPP-4 inhibitor linagliptin increases active GLP-2 and decreases colonic cytokines in a mouse inflammatory bowel disease model [abstract no. 1124-P]. Diabetes 2011; 60 Suppl. 1: A309
Schürmann C, Linke A, Engelmann-Pilger K, et al. The dipeptidyl peptidase-4 inhibitor linagliptin attenuates inflammation and accelerates epithelialization in wounds of diabetic ob/ob mice. J Pharmacol Exp Ther 2012 Jul; 342 (1): 71–80
Darsalia V, Olverling A, Ortsäter H, et al. Linagliptin reduces ischaemic brain damage following stroke in a high-fat diet mouse model: a comparison to glimepiride [abstract no. 931-P plus poster]. 72nd Scientific Sessions of the American Diabetes Association; 2012 Jun 8–12; Philadelphia (PA)
Boehringer Ingelheim International GmbH. Tradjenta™ (linagliptin) tablets: US prescribing information [online]. Available from URL: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/201280s002lbl.pdf [Accessed 2012 Jul 27]
Boehringer Ingelheim International GmbH. Trajenta 5 mg film-coated tablets; summary of product characteristics [online]. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002110/WC500115745.pdf [Accessed 2012 Jul 27]
Ring A, Port A, Graefe-Mody EU, et al. The DPP-4 inhibitor linagliptin does not prolong the QT interval at therapeutic and supratherapeutic doses. Br J Clin Pharmacol 2011 Jul; 72 (1): 39–50
Friedrich C, Glund S, Lionetti D, et al. Pharmacokinetic and pharmacodynamic evaluation of linagliptin in African American patients with type 2 diabetes [abstract]. J Clin Pharmacol 2011 Sep; 51 (9): 1336
Graefe-Mody U, Giessmann T, Ring A, et al. A randomized, open-label, crossover study evaluating the effect of food on the relative bioavailability of linagliptin in healthy subjects. Clin Ther 2011 Aug; 33 (8): 1096–103
Blech S, Ludwig-Schwellinger E, Gräfe-Mody EU, et al. The metabolism and disposition of the oral dipeptidyl peptidase-4 inhibitor, linagliptin, in humans. Drug Metab Dispos 2010 Apr; 38 (4): 667–78
Graefe-Mody U, Rose P, Retlich S, et al. Pharmacokinetics of linagliptin in subjects with hepatic impairment. Br J Clin Pharmacol 2012 Jul; 74 (1): 75–85
Graefe-Mody U, Friedrich C, Port A, et al. Effect of renal impairment on the pharmacokinetics of the dipeptidyl peptidase-4 inhibitor linagliptin. Diabetes Obes Metab 2011 Oct; 13 (10): 939–46
Friedrich C, Emser A, Woerle HJ, et al. Renal impairment has no relevant effect on long-term exposure of linagliptin in patients with type 2 diabetes mellitus [abstract no. 1105-P]. Diabetes 2011; 60 Suppl. 1: A303–4
Boehringer Ingelheim International GmbH. Jentadueto (linagliptin and metformin hydrochloride) tablets: US prescribing information [online]. Available from URL: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/201281s000lbl.pdf [Accessed 2012 Jul 27]
Fuchs H, Tillement JP, Urien S, et al. Concentration-dependent plasma protein binding of the novel dipeptidyl peptidase 4 inhibitor BI 1356 due to saturable binding to its target in plasma of mice, rats and humans. J Pharm Pharmacol 2009 Jan; 61 (1): 55–62
Graefe-Mody EU, Brand T, Ring A, et al. Effect of linagliptin on the pharmacokinetics and pharmacodynamics of warfarin in healthy volunteers. Int J Clin Pharmacol Ther 2011 May; 49 (5): 300–10
Graefe-Mody U, Huettner S, Stähle H, et al. Effect of linagliptin (BI 1356) on the steady-state pharmacokinetics of simvastatin. Int J Clin Pharmacol Ther 2010 Jun; 48 (6): 367–74
Friedrich C, Port A, Ring A, et al. Effect of multiple oral doses of linagliptin on the steady-state pharmacokinetics of a combination oral contraceptive in healthy female adults: an open-label, two-period, fixed-sequence, multiple-dose study. Clin Drug Invest 2011; 31 (9): 643–53
Graefe-Mody EU, Jungnik A, Ring A, et al. Evaluation of the pharmacokinetic interaction between the dipeptidyl peptidase-4 inhibitor linagliptin and pioglitazone in healthy volunteers. Int J Clin Pharmacol Ther 2010 Oct; 48 (10): 652–61
Graefe-Mody U, Rose P, Ring A, et al. Assessment of the pharmacokinetic interaction between the novel DPP-4 inhibitor linagliptin and a sulfonylurea, glyburide, in healthy subjects. Drug Metab Pharmacokinet 2011; 26 (2): 123–9
Graefe-Mody EU, Padula S, Ring A, et al. Evaluation of the potential for steady-state pharmacokinetic and pharmacodynamic interactions between the DPP-4 inhibitor linagliptin and metformin in healthy subjects. Curr Med Res Opin 2009 Aug; 25 (8): 1963–72
Migoya EM, Bergeron R, Miller JL, et al. Dipeptidyl peptidase-4 inhibitors administered in combination with metformin result in an additive increase in the plasma concentration of active GLP-1 [published erratum appears in Clin Pharmacol Ther 2011 Feb; 89 (2): 320]. Clin Pharmacol Ther 2010 Dec; 88 (6): 801–8
Friedrich C, Ring A, Brand T, et al. Evaluation of the pharmacokinetic interaction after multiple oral doses of linagliptin and digoxin in healthy volunteers. Eur J Drug Metab Pharmacokinet 2011 Mar; 36 (1): 17–24
Barnett AH, Harper R, Toorawa R, et al. Linagliptin monotherapy improves glycaemic control in type 2 diabetes patients for whom metformin therapy is inappropriate [abstract no. 823]. Diabetologia 2010; 53 Suppl. 1: S327
Rafeiro E, Ross SA, Meinicke T, et al. Efficacy and safety of 5 mg daily dosing regimens with linagliptin in patients with type 2 diabetes inadequately controlled on metformin [abstract no. 831]. Diabetologia 2011; 54 Suppl. 1: S338–9
Lewin AJ, Arvay L, Liu D, et al. Safety and efficacy of linagliptin as add-on therapy to a sulphonylurea in inadequately controlled type 2 diabetes [abstract no. 821]. 46th Annual Meeting of the European Association for the Study of Diabetes; 2010 Sep 20–24; Stockholm
Barnett AH, Huisman H, Jones R, et al. Efficacy and safety of linagliptin in elderly patients (≥ 70 years) with type 2 diabetes [abstract no. 1017-P plus poster]. 72nd Scientific Sessions of the American Diabetes Association; 2012 Jun 8–12; Philadelphia (PA)
Thrasher JR, Ahmed A, Daniels K, et al. Randomized, placebo-controlled, double-blind, 24-week study of linagliptin 5 mg/day in Black/African American patients with type 2 diabetes [abstract]. 21st Annual Scientific and Clinical Congress of the American Association of Clinical Endocrinologists; 2012 May 23–27; Philadelphia (PA)
Newman J, McGill JB, Patel S, et al. Long-term efficacy and safety of linagliptin in patients with type 2 diabetes and severe renal impairment [abstract no. 821]. Diabetologia 2011; 54 Suppl. 1: S333
Sloan L, Newman J, Sauce C, et al. Safety and efficacy of linagliptin in type 2 diabetes patients with severe renal impairment [abstract no. 413-PP]. Diabetes 2011 Jul; 60 Suppl.1: A114. Plus poster presented at the 71st Scientific Sessions of the American Diabetes Association; 2011 Jun 24–28; San Diego (CA)
Yki-Jarvinen H, Durán-Garcia S, Pinnetti S, et al. Efficacy and safety of linagliptin as add-on therapy to basal insulin in patients with type 2 diabetes [abstract no. 999-P plus poster]. 72nd Scientific Sessions of the American Diabetes Association; 2012 Jun 8–12; Philadelphia (PA)
Boehringer Ingelheim Pharmaceuticals. A randomised, db, placebo-controlled study of BI 1356 for 18 weeks followed by a 34 week double-blind extension period (placebo patients switched to glimepiride) in type 2 diabetic patients for whom treatment with metformin is inappropriate [Clinicaltrials.gov identifier NCT00740051]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://www.clinicaltrials.gov [Accessed 2012 Mar 22]
Boehringer Ingelheim Pharmaceuticals. Linagliptin 2.5 mg twice daily versus 5 mg once daily as add-on therapy to twice daily metformin in type 2 diabetes [ClinicalTrials.gov identifier NCT01012037]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://www.clinicaltrials.gov [Accessed 2012 Mar 21]
Boehringer Ingelheim Pharmaceuticals. Randomized, double-blind (db), placebo-controlled 18 week study of linagliptin (BI 1356) in type 2 diabetic patients with insufficient glycaemic control on a sulfonylurea drug [ClinicalTrials.gov identifier NCT00819091]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://www.clinicaltrials.gov [Accessed 2012 Mar 22]
Boehringer Ingelheim Pharmaceuticals. Efficacy and safety of linagliptin in elderly patients with type 2 diabetes [ClinicalTrials.gov identifier NCT01084005]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://www.clinicaltrials.gov [Accessed 2012 Jun 12]
Boehringer Ingelheim Pharmaceuticals. Efficacy and safety of linagliptin (BI 1356) in Black/African Americans with type 2 diabetes with a MTT sub-study [ClinicalTrials.gov identifier NCT01194830]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://www.clinicaltrials.gov [Accessed 2012 May 30]
Boehringer Ingelheim Pharmaceuticals. Safety and efficacy in type 2 diabetic patients with severe chronic renal impairment, 5 mg BI 1356 (linagliptin) vs. placebo, insulin background inclusive [ClinicalTrials.gov identifier NCT00800683]. US national Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://www.clinicaltrials.gov [Accessed 2012 Mar 28]
Patel S, Barnett AH, Harper R, et al. 1 yr linagliptin monotherapy is well tolerated & sustains improvement in glycaemic control in patients for whom metformin is inappropriate [abstract no. D-0990a]. International Diabetes Federation World Diabetes Congress; 2011 Dec 4–8; Dubai
von Eynatten M, Haak T, Meinicke T, et al. Initial combination of linagliptin and metformin in patients with type 2 diabetes mellitus: efficacy and safety in a 1-year, randomised, double-blind extension study [abstract]. 21st Annual Scientific and Clinical Congress of the American Association of Clinical Endocrinologists; 2012 May 23–27; Philadelphia (PA)
Rendell M, Chrysant S, Trujillo A, et al. Linagliptin improves glycemic control independent of body mass index in patients with type 2 diabetes [abstract]. J Gen Intern Med 2011 May; 26 Suppl. 1: S214-5
Rendell M, Chrysant SG, Emser A, et al. Linagliptin improves glycemic control independent of gender in patients with type 2 diabetes [abstract]. Endocr Rev 2011 Jun; 32 (03_meeting abstracts)
Patel S, Weber S, Emser A, et al. Linagliptin improves glycaemic control independently of diabetes duration and insulin resistance in patients with type 2 diabetes [abstract no. 832]. Diabetologia 2011 Sep; 54 Suppl. 1: S339. Plus poster presented at the 47th Annual Meeting of the European Association for the Study of Diabetes; 2011 Sep 12–16; Lisbon
Rendell M, Chrysant S, Emser A, et al. Linagliptin effectively reduces HbA1c independent of age in patients with type 2 diabetes [abstract]. Pharmacotherapy 2011 Oct; 31 (10): 337e-8e
Patel S, Von Eynatten M, Weber S, et al. Linagliptin improves glycaemic control in type 2 diabetes mellitus (T2DM) patients with increased cardiovascular (CV) risk [abstract]. Diabetes, Stoffwechsel und Herz 2011 Nov; 20 (6): 431
Gallwitz B, Rosenstock J, Emser A, et al. Linagliptin is more effective than glimepiride at achieving a composite outcome of A1c target with no hypoglycaemia and no weight gain over 2 years in mildly hyperglycaemic T2D pts on metformin [abstract no. 1044-P]. 72nd Scientific Sessions of the American Diabetes Association; 2012 Jun 8–12; Philadelphia (PA)
Zeng Z, Choi DS, Mohan V, et al. Linagliptin is efficacious and well tolerated in Asian patients with inadequately controlled type 2 diabetes [abstract no. 1163-P]. 72nd Scientific Sessions of the American Diabetes Association; 2012 Jun 8–12; Philadelphia (PA)
Groop PH, Cooper M, Perkovic V, et al. Linagliptin lowers albuminuria on top of recommended standard treatment for diabetic nephropathy [abstract no. 953-P plus poster]. 72nd Scientific Sessions of the American Diabetes Association; 2012 Jun 8–12; Philadelphia (PA)
Cooper M, Von Eynatten M, Emser A, et al. Efficacy and safety of linagliptin in patients with type 2 diabetes with or without renal impairment: results from a global phase 3 program [abstract no. 1068-P]. Diabetes 2011 Jul; 60 Suppl. 1: A293
Groop P-H, Von Eynatten M, Emser A, et al. Efficacy and safety of linagliptin in type 2 diabetes patients at high risk of renal complications: results from a large phase 3 program [abstract no. 2274-PO]. Diabetes 2011 Jul; 60 Suppl. 1: A605
von Eynatten M, Neubacher D, Stat D, et al. Safety and efficacy of linagliptin in combination with basal insulin: a pre-specified, pooled analysis in a vulnerable population of elderly patients (age ≥70 years) with type 2 diabetes [abstract no. OR17-3]. Endocr Rev 2012 Jun; 33 (03_meetingabstracts)
von Eynatten M, Gong Y, Emser A, et al. Safety and efficacy of linagliptin in type 2 diabetes patients with common renal and cardiovascular risk factors [poster no. P709]. 15th International Congress of Endocrinology and 14th European Congress of Endocrinology; 2012 May 5–9; Florence
Haak T, Meinicke T, Jones R, et al. Initial combination of linagliptin and metformin improves glycaemic control in type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab 2012 Jun; 14 (6): 565–74
Gomis R, Owens DR, Taskinen MR, et al. Long-term safety and efficacy of linagliptin as monotherapy or in combination with other glucose-lowering agents in 2121 subjects with type 2 diabetes: up to 2 years exposure in 24-week phase III trials followed by a 78-week open-label extension. Int J Clin Pract 2012 Aug; 66 (8): 731–40
Schernthaner G, Barnett AH, Emser A, et al. Safety and tolerability of linagliptin: a pooled analysis of data from randomized controlled trials in 3572 patients with type 2 diabetes mellitus. Diabetes Obes Metab 2012 May; 14 (5): 470–8
Johansen OE, Neubacher D, von Eynatten M, et al. Cardiovascular safety with linagliptin in patients with type 2 diabetes mellitus: a pre-specified, prospective, and adjudicated meta-analysis of a phase 3 programme. Cardiovasc Diabetol 2012 Jan 10; 11 (3)
Gooβen K, Gräber S. Longer term safety of dipeptidyl peptidase-4 inhibitors in patients with type 2 diabetes mellitus: systematic review and meta-analysis. Diabetes Obes Metab. Epub 2012 Apr 20
Araki E, Kawamori R, Inagaki N, et al. Long-term safety of linagliptin monotherapy in Japanese patients with type 2 diabetes [abstract no. P-1155 plus poster]. 21st World Diabetes Congress of the International Diabetes Federation; 2011 Dec 4–8; Dubai
Boehringer Ingelheim. Jentadueto® (linagliptin/metformin hydrochloride) tablets receive approval for the treatment of adults with type 2 diabetes in Europe [media release]. 2012 Jul 25 [online]. Available from URL: http://us.boehringer-ingelheim.com/news_events/press_releases/press_release_archive/2012/july_25_2012.html
Mayo Clinic. Type 2 diabetes: complications [online]. Available from URL: http://www.mayoclinic.com/health/type-2-diabetes/DS00585/DSECTION=complications [Accessed 2012 May 13]
Mattila TK, de Boer A. Influence of intensive versus conventional glucose control on microvascular and macrovascular complications in type 1 and 2 diabetes mellitus. Drugs 2010 Dec 3; 70 (17): 2229–45
American Diabetes Association. Standards of medical care in diabetes-2012. Diabetes Care 2012 Jan; 35 Suppl. 1: S11-63
Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012 Jun; 55 (6): 1577–96
Qaseem A, Humphrey LL, Sweet DE, et al. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2012 Feb 7; 156 (3): 218–31
Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract 2009 Sep–Oct; 15 (6): 540–59
Gavin JR, Freeman JS, Shubrook JH, et al. Type 2 diabetes mellitus: practical approaches for primary care physicians. J Am Osteopath Assoc 2011 May; 111 (5 Suppl. 4): S3-12
Ismail-Beigi F. Clinical practice. Glycemic management of type 2 diabetes mellitus. N Engl J Med 2012 Apr 5; 366 (14): 1319–27
Davidson JA. Incorporating incretin-based therapies into clinical practice: differences between glucagon-like peptide 1 receptor agonists and dipeptidyl peptidase 4 inhibitors. Mayo Clin Proc 2010 Dec; 85 (12 Suppl.): S27-37
Barnett AH. New treatments in type 2 diabetes: a focus on the incretin-based therapies. Clin Endocrinol (Oxf) 2009 Mar; 70 (3): 343–53
Peters AL. Patient and treatment perspectives: revisiting the link between type 2 diabetes, weight gain, and cardiovascular risk. Cleve Clin J Med 2009 Dec; 76 Suppl. 5: S20-7
Novartis Pharma GmbH. Galvus tablets: summary of product characteristics [online]. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000771/WC500020327.pdf [Accessed 2012 Jul 27]
Boehringer Ingelheim International GmbH. Tradjenta (linagliptin) tablets: US prescribing information [online]. Available from URL: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/201280s003s004lbl.pdf [Accessed 2012 Aug 21]
National Kidney Foundation. Diabetes and chronic kidney disease: stages 1–4 [online]. Available from URL: http://www.kidney.org/atoz/pdf/diabetes.pdf [Accessed 2012 May 15]
Merck Sharp & Dohme Ltd. Januvia film-coated tablets: summary of product characteristics [online]. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000722/WC500039054.pdf [Accessed 2012 Jul 27]
Merck Sharp & Dohme Corp. Januvia (sitagliptin) tablets: US prescribing information [online]. Available from URL: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021995s023lbl.pdf [Accessed 2012 Jul 27]
Bristol-Myers Squibb and AstraZeneca EEIG. Onglyza film-coated tablets: summary of product characteristics [online]. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001039/WC500044316.pdf [Accessed 2012 Jul 27]
Bristol-Myers Squibb Company and AstraZeneca Pharmaceuticals LP. Onglyza (saxagliptin) tablets: US prescribing information [online]. Available from URL: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/022350s004lbl.pdf [Accessed 2012 Jul 27]
Food and Drug Administration. Guidance for industry: diabetes mellitus — evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes [online]. Available from URL: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM071627.pdf?utm_campaign=Google2utm_source=fdaSearchutm_medium=websiteutm_term=guidanceforindustrydiabetesutm_content=1 [Accessed 2012 May 17]
European Medicines Agency. Guideline on clinical investigation of medicinal products in the treatment of diabetes mellitus [online]. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/10/WC500115945.pdf [Accessed 2012 May 17]
Yoon KH, Shockey GR, Teng R, et al. Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and pioglitazone on glycemic control and measures of β-cell function in patients with type 2 diabetes. Int J Clin Pract 2011 Feb; 65 (2): 154–64
Monami M, Dicembrini I, Martelli D, et al. Safety of dipeptidyl peptidase-4 inhibitors: a meta-analysis of randomized clinical trials. Curr Med Res Opin 2011 Nov; 27 Suppl. 3: 57-64
Rosenstock J, Marx N, Kahn SE, et al. Rationale and design of the CAROLINA trial: an active comparator CARdiOvascular Outcome study of the DPP-4 inhibitor LINAgliptin in patients with type 2 diabetes at high cardiovascular risk [abstract no. 1103-P]. 71st scientific sessions of the American Diabetes Association; 2011 Jun 24–28; San Diego (CA)
Elashoff M, Matveyenko AV, Gier B, et al. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology 2011 Jul; 141 (1): 150–6
Gross JL, Rogers J, Polhamus D, et al. A novel model-based meta-analysis to estimate comparative efficacies of two drugs: an example using the DPP-4 inhibitors linagliptin and sitagliptin in type 2 diabetes mellitus [abstract no. 994-P]. 72nd Scientific Sessions of the American Diabetes Association; 2012 Jun 8–12; Philadelphia (PA)
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: D.S.H. Bell, Southside Endocrinology, University of Alabama, Birmingham, AL, USA; G. Bertino, Department of Internal Medicine and Systemic Diseases, University of Catania-Italy Policlinic, Catania, Italy; G.T. Chew, School of Medicine and Pharmacology, University of Western Australia, Perth, WA, Australia; S. Del Prato, Department of Endocrinology and Metabolism, University of Pisa, Pisa, Italy; J. Eriksson, Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland and the Department of General Practice and Primary Health Care, University of Helsinki, Helsinki, Finland; J. Freeman, Division of Endocrinology and Metabolism, Philadelphia College of Osteopathic Medicine, Philadelphia, PA, USA.
Data Selection
Sources: Medical literature (including published and unpublished data) on ‘linagliptin’ was identified by searching databases (including MEDLINE and EMBASE) for articles published since 1996, bibliographies from published literature, clinical trial registries/databases and websites (including those of regional regulatory agencies and the manufacturer). Additional information (including contributory unpublished data) was also requested from the company developing the drug.
Search strategy: MEDLINE and EMBASE search terms were ‘linagliptin’ and (‘non insulin dependent diabetes mellitus’ or ‘type 2 diabetes mellitus’ or ‘diabetes mellitus, type 2’). Searches were last updated 23 July 2012.
Selection: Studies in patients with type 2 diabetes mellitus who received linagliptin. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Linagliptin, dipeptidyl peptidase-4 inhibitor, type 2 diabetes mellitus, pharmacodynamics, pharmacokinetics, therapeutic use, tolerability.
An erratum to this article is available at http://dx.doi.org/10.1007/s40265-013-0012-8.
Rights and permissions
About this article
Cite this article
Deeks, E.D. Linagliptin. Drugs 72, 1793–1824 (2012). https://doi.org/10.2165/11209570-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11209570-000000000-00000