Summary
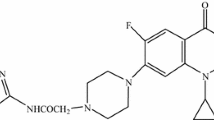
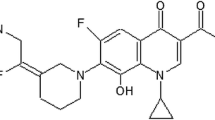
Fluoroquinolones are potent broad spectrum antibacterial agents. Two classifications have been described: chemical and biological. Quinolones can be classified into 4 groups according to their chemical structures: monocyclic, bicyclic, tricyclic and tetracyclic derivatives. Each group can be subdivided into subgroups if a fluorine atom is fixed at the 6-position.
The biological classification recognised 4 groups. Groups 1 and 2 are composed of compounds showing limited spectra (Enterobacteriaceae) and groups 3 and 4 contain compounds displaying broad antibacterial spectra. Compounds that are highly metabolised fall into groups 1 and 3 and those poorly metabolised (< 5%) into groups 2 and 4.
The structure of fluoroquinolones may help to predict antibacterial activity, pharmacokinetics, physicochemical properties, toxicity and adverse events.
Similar content being viewed by others
References
Albrecht R. Development of antibacterial agents of the nalidixic acid type. Prog Drug Res 1977; 21: 9–104
Bryskier A. Recent advances in fluoroquinolones research. Curr Opin Invest Drugs 1993; 2: 409–15
Bryskier A. Update on fluoroquinolones. Curr Opin Invest Drugs 1994; 3: 41–9
Chu DTW., Fernandes PB. Structure-activity relationships of the fluoroquinolones. Antimicrob Agents Chemother 1989; 33: 131–5
Chu DTW, Fernandes PB. Recent development in the field of quinolone antibacterial agents. Adv Drug Res 1991; 21: 44–144
Wolfson JS, Hooper DC. Fluoroquinolone antimicrobial agents. Clin Microbiol Rev 1989; 2: 378–424
Domagala JM. Structure-activity and structure-side-effect relationships for the quinolone antibacterial. J Antimicrob Chemother 1994; 33: 685–706
Ashina Y, Ishizaki T, Suzue S. Recent advances in structure activity relationships in new quinolones. Prog Drug Res 1992; 38: 57–106
Price JR. Some reactions of 1-methyl-y-quinolone-3-carboxylic acid, a degradation product of the alcaloids. Aust J Sci Res 1949; 217: 272–81
Barton N, Crowther AF, Hesworth W, et al. Quinolones and therapeutic compositions containing them. UK patent no. 830 832, March, 1960
Bryskier A. Fluoroquinolones: mechanisms of action and resistance. Int J Antimicrob Agents 1993; 2: 151–84
Inoue S, Ohue T, Yamagishi J, et al. Mode of incomplete cross-resistance among pipemidic, piromidic and nalidixic acids. Antimicrob Agents Chemother 1978; 14: 240–5
Rohlfing SR, Gerster IF, Kwam DC. Bioevaluation of the anti-bacterial fluoroquinolone for urinary tract use. Antimicrob Agents Chemother 1976; 10: 20–4
Bryskier A. New pyridone-β-carboxylic acid derivatives. Quinolones Bulletin 1993
Georgopapadakou NH, Dix BA, Angehrn P, et al. Monocyclic and tricyclic analogs of quinolones. Antimicrob Agents Chemother 1987; 31: 614–6
Bryskier A, Chantot J-F, Veyssier P. Classification and structure activity relationship of new pyridone-β-carboxylic acid derivatives. Rev Infect Dis 1988; 10 Suppl. 1: S10
Chu DTW, Nordeen CW, Hardy DJ, et al. Synthesis, antimicrobial activities, and pharmacological properties of enantiomers of temafloxacinhydrochloride. J Med Chem 1991; 34: 160–74
Bryskier A, Chantot JF, Veyssier P, et al. The classification and structure-activity relationships of pyridone-β-carboxylic acid derivatives. In: Mitsuhashi S, Daikos GK, editors. Proceedings of a Workshop at the 14th International Congress of Chemotherapy; Kyoto, 1985: 33-7
Koike M, Nii S, Morita S, et al. OPC-17116, a new quinolone: pharmacokinetics and metabolism in healthy volunteers [abstract 1482]. 31st Interscience Conference of Antimicrobial Agents and Chemotherapy; 1991 Oct 2: Chicago: 1991
Marsh K, Eiden E, Hernandez L, et al. In vitro and in vivo evaluation of a series of amino acid prodrugs of tosufloxacin [abstract 1441]. 31st Interscience Conference of Antimicrobial Agents and Chemotherapy; 1991 Oct 2: Chicago: 1991
Janknegt R. Drug interactions with quinolones. J Antimicrob Chemother 1990; 26 Suppl. D: 7–29
Hori S, Kanemitsu K, Shimada J. Convulsivant activity of DU-6859a —a newly synthesized quinolone — a comparative study on convulsivant activity of new quinolones [abstract 1002]. Proceedings of the 33rd Interscience Conference of Antimicrobial Agents and Chemotherapy; 1993: New Orleans: 1993
Matsubura S, Marutani K, Matsumoto M, et al. Reduced phototoxicity of Q-35, a new 8-methoxy fluoroquinolone-II-UVA irradiation to murine ears [abstract 1504]. Proceedings of the 33rd Interscience Conference of Antimicrobial Agents and Chemotherapy; 1993: New Orleans: 1993
Fort FL. Mutagenicity of quinolone antibacterials. Drug Saf 1992; 7: 214–22
Hoshino K, Sato K, Une T, et al. Inhibitory effects of quinolones on DNA gyrase of Escherichia coli and topoisomerase II of fetal calf thymus. Antimicrob Agents Chemother 1989; 33: 1816–8
Wijinands WJA, van Hervaerden CLA, Vree TB. Enoxacin raises plasma theophylline concentrations. Lancet 1984; 2: 108–9
Fuhr U, Strobl G, Manaut F, et al. Quinolone antibacterial agents against cytochrome P-450 A12 activity in vitro and in vivo Antimicrob Agents Chemother 1992; 36: 942–8
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bryskier, A., Chantot, JF. Classification and Structure-Activity Relationships of Fluoroquinolones. Drugs 49 (Suppl 2), 16–28 (1995). https://doi.org/10.2165/00003495-199500492-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-199500492-00005