Abstract
Objectives
To investigate the pharmacokinetics of mycophenolic acid (MPA) in Chinese adult renal allograft recipients, and to generate the validated model equations for estimation of the MPA area under the plasma concentration-time curve from 0 to 12 hours (AUC12) with a limited sampling strategy.
Patients and methods
The pharmacokinetics in 75 Chinese renal allograft recipients treated with mycophenolate mofetil 2 g/day in combination with Ciclosporin and corticosteroids were determined. The MPA concentration was assayed by high-performance liquid chromatography at pre-dose (C0) and at 0.5 (C0.5), 1 (C1), 1.5 (C1.5), 2 (C2), 4 (C4), 6 (C6), 8 (C8), 10 (C10) and 12 (C12) hours after dosing on day 14 post-transplant. Patients were randomly divided into: (i) a model group (n = 50) to generate the model equations by multiple stepwise regression analysis for estimation of the MPA AUC by a limited sampling strategy; and (ii) a validation group (n = 25) to evaluate the predictive performance of the model equations.
Results
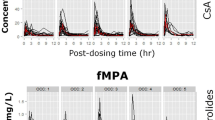
The mean MPA AUC12 was 52.97 ± 15.09 mg · h/L, ranging from 24.0 to 102.3 mg · h/L. The patient’s age and serum albumin level had a significant impact on the MPA AUC12. The correlation between the pre-dose MPA trough level (C0) and the MPA AUC12 was poor (r2 = 0.02, p = 0.33). Model equations 7 (MPA AUC12 = 14.81 ± 0.80 · C0.5 ± 1.56 · C2 ± 4.80 · C4, r2 = 0.70) and 11 (MPA AUC12 = 11.29 ± 0.51 · C0.5 ± 2.13 · C2 ± 8.15 · C8, r2 = 0.88) were selected for MPA AUC calculation in Chinese patients, resulting in good agreements between the estimated MPA AUC and the full MPA AUC12, with a mean prediction error of ±10.1 and ±6.9 mg · h/L, respectively.
Conclusion
In Chinese renal allograft recipients, MPA pharmacokinetics manifest substantial interindividual variability, and the MPA AUC12 tends to be higher than that in Caucasian patients receiving the same dose of mycophenolate mofetil. Two validated model equations with three sampling timepoints are recommended for MPA AUC estimation in Chinese patients.








Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Lipsky JJ. Mycophenolate mofetil. Lancet 1996; 348: 1357–9
Fulton B, Markham A. Mycophenolate mofetil: a review of its pharmacodynamic and pharmacokinetic properties and clinical efficacy in renal transplantation. Drugs 1996; 51: 278–98
European Mycophenolate Mofetil Cooperative Study Group. Placebo-controlled study of mycophenolate mofetil combined with cyclosporine and corticosteroids for prevention of acute rejection. Lancet 1995; 345: 1321–5
Allison AC, Eugui EM. Immunosuppressive and other effects of mycophenolic acid and an ester prodrug, mycophenolate mofetil. Immunol Rev 1993; 136: 5–28
Sievers TM, Rossi SJ, Ghobrial RM, et al. Mycophenolate mofetil. Pharmacotherapy 1997; 17: 1178–97
Sollinger HW, US Renal Transplant Mycophenolate Mofetil Study Group. Mycophenolate mofetil for the prevention of acute rejection in primary cadaveric renal allograft recipients. Transplantation 1995; 60: 225–32
The Tricontinental Mycophenolate Mofetil Renal Transplantation Study Group. A blinded, randomized clinical trial of mycophenolate mofetil for the prevention of acute rejection in cadaveric renal transplantation. Transplantation 1996; 61: 1029–37
Meier-Kriesche HU, Steffen BJ, Hochberg AM, et al. Long term use of mycophenolate mofetil is associated with a reduction in the incidence and risk of late rejection. Am J Transplant 2003; 3: 68–73
Meier-Kriesche HU, Steffen BJ, Hochberg AM, et al. Mycophenolate mofetil versus azathioprine therapy is associated with a significant protection against long-term renal allograft function deterioration. Transplantation 2003; 75: 1341–6
Shaw LM, Nicholls A, Hale M, et al. Therapeutic monitoring of mycophenolic acid: a consensus panel report. Clin Biochem 1998; 31: 317–22
Appel GB, Radhakrishnan J, Ginzler EM. Use of mycophenolate mofetil in autoimmune and renal diseases. Transplantation 2005; 80 (2 Suppl.): S265–71
Maes BD, Oyen R, Claes K, et al. Mycophenolate mofetil in IgA nephropathy: results of a 3-year prospective placebo-controlled randomized study. Kidney Int 2004; 65: 1842–9
Cattaneo D, Gaspari F, Ferrari S, et al. Pharmacokinetics help optimizing mycophenolate mofetil dosing in kidney transplant patients. Clin Transplant 2001; 15: 402–9
Atcheson BA, Taylor PJ, Mudge DW, et al. Mycophenolic acid pharmacokinetics and related outcomes early after renal transplant. Br J Clin Pharmacol 2005; 59(3): 271–80
Kiberd BA, Lawen J, Fraser AD, et al. Early adequate mycophenolic acid exposure is associated with less rejection in kidney transplantation. Am J Transplant 2004; 4: 1079–83
Kuypers DR, Claes K, Evenepoel P, et al. Clinical efficacy and toxicity profile of tacrolimus and mycophenolic acid in relation to combined long-term pharmacokinetics in de novorenal allograft recipients. Clin Pharmacol Ther 2004; 75: 434–47
van Gelder T, Hilbrands LB, Vanrenterghem Y, et al. A randomized double-blind, multicenter plasma concentration controlled study of the safety and efficacy of oral mycophenolate mofetil for the prevention of acute rejection after kidney transplantation. Transplantation 1999; 68: 261–6
Weber LT, Shipkova M, Armstrong VW, et al. The pharmacokinetic-pharmacodynamic relationship for total and free mycophenolic acid in pediatric renal transplant recipients: a report of the German Study Group on Mycophenolate Mofetil Therapy. J Am Soc Nephrol 2002; 13: 759–68
Shaw LM, Korecka M, Aradhye S, et al. Mycophenolic acid area under the curve values in African American and Caucasian renal transplant patients are comparable. J Clin Pharmacol 2000; 40: 624–33
Bullingham RE, Nicholls A, Hale M. Pharmacokinetics of mycophenolate mofetil (RS61443): a short review. Transplant Proc 1996; 28: 925–9
Filler G, Zimmering M, Mai I. Pharmacokinetics of mycophenolate mofetil are influenced by concomitant immunosuppression. Pediatr Nephrol 2000; 14: 100–4
Pisupati J, Jain A, Burckart G, et al. Intraindividual and interindividual variations in the pharmacokinetics of mycophenolic acid in liver transplant patients. J Clin Pharmacol 2005; 45: 34–41
Bennett WM. Immunosuppression with mycophenolic acid: one size does not fit all. J Am Soc Nephral 2003; 14: 2414–6
Oellerich M, Shipkova M, Schutz E, et al. Pharmacokinetic and metabolic investigations of mycophenolic acid in pediatric patients after renal transplantation: implications for therapeutic drug monitoring. German Study Group on Mycophenolate Mofetil Therapy in pediatric Renal Transplant Recipients. Ther Drug Monit 2000; 22: 20–6
Shaw LM, Korecka M, DeNofrio D, et al. Pharmacokinetic, pharmacodynamic, and outcome investigations as the basis for mycophenolic acid therapeutic drug monitoring in renal and heart transplant patients. Clin Biochem 2001; 34: 17–22
Yamani MH, Starling RC, Goormastic M, et al. The impact of routine mycophenolate mofetil drug monitoring on the treatment of cardiac allograft rejection. Transplantation 2000; 69: 2326–30
Cox VC, Ensom MH. Mycophenolate mofetil for solid organ transplantation: does the evidence support the need for clinical pharmacokinetic monitoring? Ther Drug Monit 2003; 25: 137–57
Shaw LM, Holt DW, Oellerich M, et al. Current issues in therapeutic drug monitoring of mycophenolic acid: report of a roundtable discussion. Ther Drug Monit 2001; 23: 305–15
Pawinski T, Hale M, Korecka M, et al. Limited sampling strategy for the estimation of mycophenolic acid area under the curve in adult renal transplant patients treated with concomitant tacrolimus. Clin Chem 2002; 48: 1497–504
Filler G. Abbreviated mycophenolic acid AUC from CO, C1, C2, and C4 is preferable in children after renal transplantation on mycophenolate mofetil and tacrolimus therapy. Transpl Int 2004; 17: 120–5
Willis C, Taylor PJ, Salm P, et al. Evaluation of limited sampling strategies for estimation of 12-hour mycophenolic acid area under the plasma concentration-time curve in adult renal transplant patients. Ther Drug Monit 2000; 22: 549–54
Le Guellec C, Buchler M, Giraudeau B, et al. Simultaneous estimation of cyclosporin and mycophenolic acid areas under the curve in stable renal transplant patients using a limited sampling strategy. Eur J Clin Pharmacol 2002; 57: 805–11
Premaud A, Le Meur Y, Debord J, et al. Maximum a posteriori Bayesian estimation of mycophenolic acid pharmacokinetics in renal transplant recipients at different postgrafting periods. Ther Drug Monit 2005; 27: 354–61
van Hest R, Mathot R, Vulto A, et al. Predicting the usefulness of therapeutic drug monitoring of mycophenolic acid: a computer simulation. Ther Drug Monit 2005; 27: 163–7
Le Guellec C, Bourgoin H, Buchler M, et al. Population pharmacokinetics and Bayesian estimation of mycophenolic concentration in stable renal transplant patients. Clin Pharmacokinet 2004; 43: 253–66
He BL, Han XW, Liu J, et al. MMF and CyA in the prevention of early acute rejection after renal transplantation. Chin J Surg 2000; 38: 683–5
Wu JY, Chen JH, Wang YM, et al. Dose optimization of mycophenolate mofetil combined with cyclopurine A and Prednisone in renal transplant recipients. Chin J Nephral 2004; 20: 132–5
Cho EK, Han DJ, Kim SC, et al. Pharmacokinetic study of mycophenolic acid in Korean kidney transplant patients. J Clin Pharmacol 2004; 44: 743–50
Schweitzer EJ, Yoon S, Fink J, et al. Mycophenolate mofetil reduces the risk of acute rejection less in African-American than in Caucasian kidney recipients. Transplantation 1998; 65: 242–8
Neylan JF, US Renal Transplant Mycophenolate Mofetil Study Group. Immunosuppressive therapy in high-risk transplant patients: dose-dependent efficacy of mycophenolate mofetil in African-American renal allograft recipients. Transplantation 1997; 64: 1277–82
Yu ZC, Cai WM, Xu D, et al. HPLC determination of mycophenolate mofetil and its active metabolite mycophenolic acid in human plasma. Chin J Pharm Anal 2005; 25: 381–4
Sheiner LB, Beal SL. Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm 1981; 9: 503–12
Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; 1: 307–10
Bullingham RE, Nicholls AJ, Kamm BR. Clinical pharmacokinetics of mycophenolate mofetil. Clin Pharmacokinet 1998; 34: 429–55
Touw DJ, Neef C, Thomson AH, et al. for the Cost-Effectiveness of Therapeutic Drug Monitoring Committee of the International Association for Therapeutic Drug Monitoring and Clinical Toxicology. Cost-effectiveness of therapeutic drug monitoring: a systematic review. Ther Drug Monit 2005; 27: 10–7
Srinivas TR, Meier-Kriesche HU, Kaplan B. Pharmacokinetic principles of immunosuppressive drugs. Am J Transplant 2005; 5: 207–17
Johnston A, Holt DW. Immunosuppressant drugs: the role of therapeutic drug monitoring. Br J Clin Pharmacol 2001; 52 Suppl.: 61S–73S
Kuypers DR. Immunosuppressive drug monitoring: what to use in clinical practice today to improve renal graft outcome. Transpl Int 2005; 18: 140–50
van Gelder T, Le Meur Y, Shaw LM, et al. Therapeutic drug monitoring of mycophenolate mofetil in transplantation. Ther Drug Monit 2006; 28: 145–54
Zucker K, Rosen A, Tsaroucha A, et al. Unexpected augmentation of mycophenolic acid pharmacokinetics in renal transplant patients receiving tacrolimus and mycophenolate mofetil in combination therapy, and analogous in vitro findings. Transpl Immunol 1997; 5: 225–32
Johnson AG, Rigby RJ, Taylor PJ, et al. The kinetics of mycophenolic acid and its glucuronide metabolite in adult kidney transplant recipients. Clin Pharmacol Ther 1999; 66: 492–500
Wollenberg K, Krumme B, Pisarski P, et al. Pharmacokinetics of mycophenolic acid in the early period after kidney transplantation. Transplant Proc 1998; 30: 4090–1
Cattaneo D, Perico N, Gaspari F, et al. Glucocorticoids interfere with mycophenolate mofetil bioavailability in kidney transplantation. Kidney Int 2002; 62: 1060–7
van Gelder T, Shaw LM. The rationale for and limitations of therapeutic drug monitoring for mycophenolate mofetil in transplantation. Transplantation 2005; 80 (2 Suppl.): S244–53
Bernard O, Guillemette C. The main role of UGT1A9 in the hepatic metabolism of mycophenolic acid and the effects of naturally occurring variants. Drug Metab Dispos 2004; 32: 775–8
Kuypers DR, Naesens M, Vermeire S, et al. Single-nucleotide polymorphisms T-275A and C-2152T on early mycophenolic acid dose-interval exposure in de novo renal allograft recipients. Clin Pharmacol Ther 2005; 78: 351–61
Kuypers DR, Claes K, Evenepoel P, et al. Long-term changes in mycophenolic acid exposure in combination with tacrolimus and corticosteroids are dose dependent and not reflected by trough plasma concentration: a prospective study in 100 de novo renal allograft recipients. J Clin Pharmacol 2003; 43: 866–80
Taylor PJ, Willis C, Salm P, et al. Accurate estimation of mycophenolic acid AUC. Ther Drug Monit 2001; 23: 301–2
Huang LP, Yang SY, Wu XT, et al. Clinical observation on the early application of cellcept following cadaveric renal transplantation. Chin J Organ Transplant 2000; 21: 294–5
Ma JJ, Yu LX, Xu J, et al. Mycophenolate mofetil for prevention and treatment of rejection of graft and its safety in transplant recipients. Chin J Organ Transplant 2000; 21: 19–21
Wang YR, Wang XF, Liu YL, et al. Clinical analysis of adverse reactions caused by mycophenolate mofetil. Central South Pharmacy 2004; 2: 368–9
Qiu S, Liu L, Guo SY, Qiu s, et al. Efficacy and safety of lowdose mycophenolate mofetil after renal transplantation. Chin Pharmacist 2006; 9: 422–3
Acknowledgements
The authors would like to thank Dr Ma Tao for performing the statistical analysis. The authors did not receive any funding for this study. The authors have no conflicts of interest that are directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Zhou, PJ., Xu, D., Yu, ZC. et al. Pharmacokinetics of Mycophenolic Acid and Estimation of Exposure Using Multiple Linear Regression Equations in Chinese Renal Allograft Recipients. Clin Pharmacokinet 46, 389–401 (2007). https://doi.org/10.2165/00003088-200746050-00002
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-200746050-00002