Abstract
Objective: To characterise the pharmacokinetic-pharmacodynamic relationships for linezolid efficacy.
Design and study population: Retrospective nonblinded analysis of severely debilitated adult patients with numerous comorbid conditions and complicated infections enrolled under the manufacturers’s compassionate use programme.
Methods: Patients received intravenous or oral linezolid 600mg every 12 hours. Plasma concentrations were obtained and a multicompartmental pharmacokinetic model was fitted. Numerical integration of the fitted functions provided the area under the concentration-time curve over 24 hours (AUC), the ratio of AUC to minimum inhibitory concentration (AUC/MIC) and the percentage of time that plasma concentrations exceeded the MIC (%T>MIC).
Main outcome measures: Modelled pharmacodynamic outcomes of efficacy included probabilities of eradication and clinical cure (multifactorial logistic regression, nonparametric tree-based modelling, nonlinear regression) and time to bacterial eradication (Kaplan-Meier and Cox proportional hazards regression). Factors considered included AUC/MIC, %T>MIC, site of infection, bacterial species and MIC, and other medical conditions.
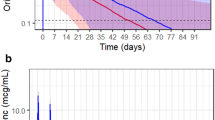
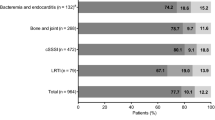
Results: There were 288 cases evaluable by at least one of the efficacy outcomes. Both %T>MIC and AUC/MIC were highly correlated (Spearman r2 = 0.868). In our analyses, within specific infection sites, the probability of eradication and clinical cure appeared to be related to AUC/MIC (eradication: bacteraemia, skin and skin structure infection [SSSI], lower respiratory tract infection [LRTI], bone infection; clinical cure: bacteraemia, LRTI) and %T>MIC (eradication: bacteraemia, SSSI, LRTI; clinical cure: bacteraemia, LRTI). Time to bacterial eradication for bacteraemias appeared to be related to the AUC, %T>MIC and AUC/ MIC. For most sites, AUC/MIC and %T>MIC models performed similarly. Conclusions: Higher success rates for linezolid may occur at AUC/MIC values of 80–120 for bacteraemia, LRTI and SSSI. Chance of success in bacteraemia, LRTI and SSSI also appear to be higher when concentrations remain above the MIC for the entire dosing interval.











Similar content being viewed by others
Notes
Use of tradenames is for product identification only and does not imply endorsement.
References
Ashtekar DR, Costa-Periera R, Shrinivasan T, et al. Oxazolidinones, a new class of synthetic antituberculosis agent: in vitro and in vivo activities of dup-721 against Mycobacterium tuberculosis. Diagn Microbiol Infect Dis 1991 Nov–Dec; 14(6): 465–71
Dresser LD, Rybak MJ. The pharmacologic and bacteriologic properties of oxazolidinones, a new class of synthetic antimicrobials. Pharmacotherapy 1998 May–Jun; 18(3): 456–62
Jernigan DB, Cetron MS, Breiman RF. Defining the public health impact of drug-resistant Streptococcus pneumoniae: report of a working group. MMWR Morb Mortal Wkly Rep 1996; 45(RR-1): 1–20
Jones RN, Johnson DM, Erwin ME. In vitro antimicrobial activities and spectra of u-100592 and u-100766, two novel fluorinated oxazolidinones. Antimicrob Agents Chemother 1996; 40(3): 720–6
Wooton M, Howe RA, Walsh TR. In-vitro activity of a range of old and new antimicrobials to hetero-vancomycin-intermediate staphylococcus aureus [abstract 2313]. 40th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2000 Sep 17–20; Toronto
Welshman IR, Sisson TA, Jungbluth GL, et al. Linezolid absolute bioavailability and the effect of food on oral bioavailability. Biopharm Drug Dispos 2001; 22(3): 91–7
Stalker D, Wajsczuk CP, Batts DH. Linezolid safety, tolerance, and pharmacokinetics after intravenous dosing twice daily for 7.5 days [abstract A-116]. 37th Interscience Conference on Antimicrobial Agents and Chemotherapy; 1997 Sep 28–Oct 1; Toronto
Stalker D, Wajsczuk CP, Batts DH. Linezolid safety, tolerance, and pharmacokinetics following oral dosing twice daily for 14.5 days [abstract A-115]. 37th Interscience Conference on Antimicrobial Agents and Chemotherapy; 1997 Sep 28–Oct 1; Toronto
Slatter JG, Stalker DJ, Feenstra KL, et al. Pharmacokinetics, metabolism, and excretion of linezolid following an oral dose of [(14)C] linezolid to healthy human subjects. Drug Metab Dispos 2001; 29(8): 1136–45
Meagher AK, Forrest A, Rayner CR, et al. Population pharmacokinetics of linezolid in patients treated in a compassionate-use program. Antimicrob Agents Chemother 2003; 47(2): 548–53
Zyvox® (linezolid) [package insert]. Kalamazoo, Michigan: Pharmacia, 2002
Bostic GD, Perri MB, Thal LA, et al. Comparative in vitro and bactericidal activity of oxazolidinone antibiotics against multidrug-resistant enterococci. Diagn Microbiol Infect Dis 1998; 30(2): 109–12
Gunderson BW, Ibrahim KH, Peloquin CA, et al. Comparison of linezolid activities under aerobic and anaerobic conditions against methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus faecium. Antimicrob Agents Chemother 2003; 47(1): 398–9
Forrest A, Nix DE, Ballow CH, et al. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob Agents Chemother 1993; 37(5): 1073–81
Thomas JK, Forrest A, Bhavnani SM, et al. Pharmacodynamic evaluation of factors associated with the development of bacterial resistance in acutely ill patients during therapy. Antimicrob Agents Chemother 1998; 42(3): 521–7
Andes D, van Ogtrop ML, Peng J, et al. In vivo pharmacodynamics of a new oxazolidinone (linezolid). Antimicrob Agents Chemother 2002; 46(11): 3484–9
Jacqueline C, Batard E, Perez L, et al. In vivo efficacy of continuous infusion versus intermittent dosing of linezolid compared to vancomycin in a methicillin-resistant Staphylococcus aureus rabbit endocarditis model. Antimicrob Agents Chemother 2002; 46(12): 3706–11
Hyatt JM, Ballow CH, Forrest A. Safety and efficacy of linezolid (pnu-100766) in eradication of nasal Staphylococcus aureus [abstract A-4]. 38th Interscience Conference on Antimicrobial Agents and Chemotherapy; 1998; San Diego (CA)
Birmingham MC, Rayner CR, Meagher AK, et al. Linezolid for the treatment of multidrug-resistant, gram-positive infections: experience from a compassionate-use program. Clin Infect Dis 2003; 36(2): 159–68
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16(1): 31–41
McCabe WR, Jackson GG. Gram-negative bacteremia. Arch Intern Med 1962; 110: 847–55
Steimer JL, Mallet A, Golmard JL, et al. Alternative approaches to estimation of population pharmacokinetic parameters: comparison with the nonlinear mixed-effect model. Drug Metab Rev 1984; 15(1–2): 265–92
Ds’Argenio DZ, Schumitzky A. A program package for simulation and parameter estimation in pharmacokinetic systems. Comput Programs Biomed 1979; 9(2): 115–34
Ds’Argenio DZ, Schumitzky A. Adapt II users’s guide: pharmacokinetic-pharmacodynamic systems analysis software. Los Angeles (CA): Biomedical Simulations Resource, 1997
Wilkinson L. Systat: the system for statistics. Chicago (IL): SPSS Inc, 1998
Akaike H. A Bayesian extension of the minimum AIC procedure of autoregressive model fitting. Biometrika 1979; 66: 237–42
Cammarata S, Hafkin B, Todd WM. Efficacy of linezolid in community acquired S. pneumoniae pneumonia [abstract]. Am J Respir Crit Care Med 1999, 159 (S)
Rubinstein E, Cammarata S, Oliphant T, et al. Linezolid (pnu-100766) versus vancomycin in the treatment of hospitalized patients with nosocomial pneumonia: a randomized, double-blind, multicenter study. Clin Infect Dis 2001; 32(3): 402–12
Cammarata S, Hafkin B, Demke DM. Efficacy of linezolid in skin and soft tissue infections [abstract]. Clin Microbiol Infect 1999, 5 (S)
Stevens DL, Herr D, Lampiris H, et al. Linezolid versus vancomycin for the treatment of methicillin-resistant Staphylococcus aureus infections. Clin Infect Dis 2002; 34(11): 1481–90
Stevens DL, Smith LG, Bruss JB, et al. Randomized comparison of linezolid (pnu-100766) versus oxacillin-dicloxacillin for treatment of complicated skin and soft tissue infections. Antimicrob Agents Chemother 2000; 44(12): 3408–13
Data on file, Pharmacia and Upjohn, 1999
Rayner CR, Forrest A, Meagher A, et al. Population (pop) pharmacodynamics (pd) of linezolid (1) in seriously-ill adult patients (pts) from a compassionate-use protocol [abstract 1390]. 40th Interscience Congress on Antimicrobial Agents and Chemotherapy; Toronto; 2000
Acknowledgements
Dr Birmingham was the principal coordinator of the compassionate use programme while at the Clinical Pharmacokinetics Laboratory until January 2001, and served as a consultant to Pharmacia Corporation. Drs Rayner, Forrest, Meagher, and Schentag also served as consultants to Pharmacia Corporation. The Clinical Pharmacokinetics Laboratory received support for the conduct of the programme from Pharmacia and UpJohn Co., Kalamazoo, MI. Informed consent was obtained from the patients or their guardians, and guidelines for human experimentation of the US Department of Health and Human Services and/or those of the investigators’ institutions were followed in the conduct of this clinical research.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rayner, C.R., Forrest, A., Meagher, A.K. et al. Clinical Pharmacodynamics of Linezolid in Seriously Ill Patients Treated in a Compassionate Use Programme. Clin Pharmacokinet 42, 1411–1423 (2003). https://doi.org/10.2165/00003088-200342150-00007
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-200342150-00007