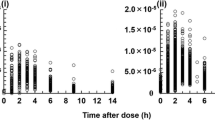
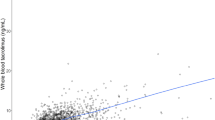
Abstract
Cyclosporin is an immunosuppressive agent with a narrow therapeutic index. The total concentration of cyclosporin in blood is usually monitored to guide dosage adjustment and to compensate for substantial interindividual and intraindividual variability in cyclosporin pharmacokinetics. Cyclosporin is a highly lipophilic molecule and widely distributes into blood, plasma and tissue components. It mainly accumulates in fat-rich organs, including adipose tissue and liver. In blood, it binds to erythrocytes in a saturable fashion that is dependent on haematocrit, temperature and the concentration of plasma proteins. In plasma, it binds primarily to lipoproteins, including high-density, low-density and very-low-density lipoprotein, and, to a lesser extent, albumin. The unbound fraction of cyclosporin in plasma (CsAfu) expressed as a percentage is approximately 2%.
It has been shown that both the pharmacokinetic and pharmacodynamic properties of cyclosporin are related to its binding characteristics in plasma. Furthermore, there is some evidence to indicate that the unbound concentration of cyclosporin (CsAU) has a closer association with both kidney and heart allograft rejection than the total (bound + unbound) concentration. However, the measurement of CsAfu is inherently complex and cannot easily be performed in a clinical setting. Mathematical models that calculate CsAfu, and hence CsAU, from the concentration of plasma lipoproteins may be a more practical option, and should provide a more accurate correlate of effectiveness and toxicity of this drug in transplant recipients than do conventional monitoring procedures.
In conclusion, the distribution characteristics of cyclosporin in blood, plasma and various tissues are clinically important. Further investigations are needed to verify whether determination of CsAU improves the clinical management of transplant recipients.






Similar content being viewed by others
Notes
Use of tradenames is for product identification only and does not imply endorsement.
References
Lindholm A. Cyclosporine A: clinical experience and therapeutic drug monitoring. Ther Drug Monit 1995; 17: 631–7
du Souich P, Verges J, Erill S. Plasma protein binding and pharmacological response. Clin Pharmacokinet 1993; 24: 435–40
Rowland M. Protein binding and drug clearance. Clin Pharmacokinet 1984; 9: 10–7
Eichler H-G, Muller M. Drug distribution, the forgotten relative in clinical pharmacokinetics. Clin Pharmacokinet 1998; 34: 95–9
Wegner R. Synthesis of cyclosporin and analogues: structure activity relationships of new cyclosporin derivatives. Transplant Proc 1983; 15 Suppl. 1: 2230–41
Cefalu WT, Pardridge WM. Restrictive transport of a lipid-soluble peptide (cyclosporin) through the blood-brain barrier. J Neurochem 1985; 45: 1954–6
Rosenthaler J, Keller HP. Comment on cyclosporine assay techniques: an attempt for recommendations. Transplant Proc 1990; 22: 1160–5
Ismailos G, Repas C, Dressman JB, et al. Unusual solubility behaviour of cyclosporin A in aqueous media. J Pharm Pharmacol 1991; 43: 287–9
Kahan BD. Cyclosporine. N Engl J Med 1989; 321: 1725–38
Di Padova FE. Pharmacology of cyclosporine (Sandimmune). V. Pharmacological effects on immune function: in vitro studies. Pharmacol Rev 1990; 41: 373–405
Handschumacher RE, Harding MW, Rice J, et al. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science 1984; 226: 544–7
Liu J, Farmer Jr JD, Lane WS, et al. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP- FK506 complexes. Cell 1991; 66: 807–15
Fahr A. Cyclosporin clinical pharmacokinetics. Clin Pharmacokinet 1993; 24: 472–95
Friman S, Backman L. A new microemulsion formulation of cyclosporin, pharmacokinetic and clinical features. Clin Pharmacokinet 1996; 30: 181–93
Prueksaritanont T, Correia MA, Rettie AE, et al. Cyclosporin metabolism by rat liver microsomes, evidence for involvement of enzyme(s) other than cytochromes P-450 3A. Drug Metab Dispos 1993; 21: 730–7
Kronbach T, Fischer V, Meyer UA. Cyclosporine metabolism in human liver: identification of a cytochrome P-450III gene family as the major cyclosporine-metabolizing enzyme explains interactions of cyclosporine with other drugs. Clin Pharmacol Ther 1988; 43: 630–5
Venkataramanan R, Starzl T, Yang S. Biliary excretion of cyclosporine in liver transplant patients. Transplant Proc 1985; 17 Suppl. 2: 286–9
Bleck JS, Schlitt HJ, Christians U, et al. Urinary excretion of ciclosporin and 17 of its metabolites in renal allograft recipients. Pharmacology 1989; 39: 160–4
Cooney GF, Habucky K, Hoppu K. Cyclosporin pharmacokinetics in paediatric transplant recipients. Clin Pharmacokinet 1997; 32: 481–95
Boland J, Atkinson K, Britton K, et al. Tissue distribution and toxicity of cyclosporin A in the mouse. Pathology 1984; 16: 117–23
Atkinson K, Biggs JC, Britton K. Distribution and persistence of cyclosporin in human tissues. Lancet 1982; II: 1165
Lensmeyer GL, Wiebe DA, Carlson IH, et al. Concentrations of cyclosporin A and its metabolites in human tissues postmortem. J Anal Toxicol 1991; 15: 110–5
Kawai R, Mathew D, Tanaka C, et al. Physiologically based pharmacokinetics of cyclosporine A: extension to tissue distribution kinetics in rats and scale-up to human. J Pharmacol Exp Ther 1998; 287: 457–68
Sangalli L, Bortolotti A, Passerini F, et al. Placental transfer, tissue distribution, and pharmacokinetics of cyclosporine in the pregnant rabbit. Drug Metab Dispos 1990; 18: 102–6
Hanas E, Larsson E, Fellstrom B, et al. Safety aspects and diagnostic findings of serial renal allograft biopsies, obtained by an automatic technique with a midsize needle. Scand J Urol Nephrol 1992; 26: 413–20
Sandborn WJ, Lawson GM, Krom RA, et al. Hepatic allograft cyclosporine concentration is independent of the route of cyclosporine administration and correlates with the occurrence of early cellular rejection. Hepatology 1992; 15: 1086–91
Lemaire M, Tillement JP. Role of lipoproteins and erythrocytes in the in vitro binding and distribution of cyclosporine A in the blood. J Pharm Pharmacol 1982; 34: 715–8
Rosano TG. Effect of hematocrit on cyclosporine (cyclosporine A) in whole blood and plasma of renal-transplant patients. Clin Chem 1988; 31: 410–2
Legg B, Rowland M. Saturable binding of cyclosporin A to erythrocytes: estimation of binding parameters in renal transplant patients and implications for bioavailability assessment. Pharm Res 1998; 5: 80–5
Atkinson K, Britton K, Briggs J. Distribution and concentration of cyclosporin in human blood. J Clin Pathol 1984; 37: 1167–71
Reichel C, von Falkenhausen M, Dengler HJ. Characterization of cyclosporine A uptake in human erythrocytes. Eur J Clin Pharmacol 1994; 46: 417–9
Shibata N, Shimakawa H, Minouchi T, et al. Erythrocyte uptake and protein binding of cyclosporin A (CyA) in human blood: factors affecting CyA concentration in erythrocytes. Biol Pharm Bull 1993; 16: 702–7
Agarwal RP, McPherson RA, Threatte GA. Evidence of cyclosporine-binding protein in human erythrocytes. Transplantation 1986; 42: 627–32
Agarwal RP, Threatte GA, McPherson RA. Temperature-dependent binding of cyclosporine to an erythrocyte protein. Clin Chem 1987; 33: 481–5
Wenk M, Follath F. Temperature dependency of apparent cyclosporin A concentrations in plasma [letter]. Clin Chem 1983; 29: 1865
Lensmeyer GL, Weibe DA, Carlson IH. Distribution of cyclosporin A metabolites among plasma and cells in whole blood: effect of temperature, hematocrit, and metabolite concentration. Clin Chem 1989; 35: 56–63
Akagi H, Reynolds A, Hjelm M. Cyclosporin A and its metabolites, distribution in blood and tissues. J Int Med Res 1991; 19: 1–18
Sgoutas D, Macmahon W, Love A, et al. Interaction of cyclosporin A with human lipoproteins. J Pharm Pharmacol 1986; 38: 583–8
Gurecki J, Warty V, Sanghvi A. The transport of cyclosporine in association with plasma lipoproteins in heart and liver transplant patients. Transplant Proc 1985; 17: 1997–2002
Andrade RJ, Lucena MI, Gonzalez-Correa JA, et al. Effect of experimental bile duct ligation on distribution of cyclosporine A among plasma lipoproteins. Transplant Proc 1993; 25: 2973–7
Herve F, Urien S, Albengres E, et al. Drug binding in plasma: a summary of recent trends in the study of drug and hormone binding. Clin Pharmacokinet 1994; 26: 44–58
Converse CA, Skinner ER. Lipoprotein separation and analysis for clinical studies. In: Mackness MI, Durrington PN, editors. Lipoprotein analysis, a practical approach. Oxford: Oxford University Press, 1992: 1–39
Niederberger W, Lemaire M, Mauer G, et al. Distribution and binding of cyclosporine in blood and tissues. Transplant Proc 1983; 15: 2419–21
Marz W, Reble B, Kemkes BM, et al. The role of lipoproteins in exchange and transfer of cyclosporine-results from in vitro investigations. Transplant Proc 1986; 18: 1281–4
Hughes TA, Gaber AO, Montgomery CE. Plasma distribution of cyclosporine within lipoproteins and ‘in vitro’ transfer between very-low-density lipoproteins, low-density lipoproteins, and high density-density lipoproteins. Ther Drug Monit 1991; 13: 289–95
Brunner LJ, Luke DR, Lautersztain J, et al. Single-dose cyclosporine pharmacokinetics in various biological fluids of patients receiving allogeneic marrow transplantation. Ther Drug Monit 1990; 12: 134–8
Luke DR, Brunner LJ, Lopez-Berestein G, et al. Pharmacokinetics of cyclosporine in bone marrow transplantation: longitudinal characterization of drug in lipoprotein fractions. J Pharm Sci 1992; 81: 208–11
Gardier AM, Mathe D, Guedeney X, et al. Effects of plasma lipid levels on blood distribution and pharmacokinetics of cyclosporin A. Ther Drug Monit 1993; 15: 274–80
Wasan KM, Pritchard PH, Ramaswamy M, et al. Differences in lipoprotein lipid concentration and composition modify the plasma distribution of cyclosporine. Pharm Res 1997; 14: 1614–21
Legg B, Rowland M. Cyclosporin: measurement of fraction unbound in plasma. J Pharm Pharmacol 1987; 39: 599–603
Zaghloul I, Ptachcinski RJ, Burckart GJ, et al. Blood protein binding of cyclosporine in transplant patients. J Clin Pharmacol 1987; 27: 240–2
Lindholm A, Henricsson S, Gang P. The free fraction of cyclosporine in plasma: clinical findings with a new method. Transplant Proc 1988; 20: 377–81
Akhlaghi F, Ashley JJ, Brown K, et al. Cyclosporine plasma unbound fraction in heart and lung transplantation recipients. Ther Drug Monit 1999; 21: 8–16
Yang H, Elmquist WF. The binding of cyclosporin A to human plasma: an in vivo microdialysis study. Pharm Res 1996; 13: 622–7
Lindholm A. Monitoring of the free concentration of cyclosporin in plasma in man. Eur J Clin Pharmacol 1990; 40: 571–5
Legg B, Rowland M. Cyclosporin: erythrocyte binding and an examination of its use to estimate unbound concentration. Ther Drug Monit 1988; 10: 16–9
Fois R, Ashley JJ. Drug binding to apparatus: a factor controlling time to equilibrium in equilibrium dialysis studies. J Pharm Sci 1991; 80: 300–2
Henricsson S. A new method for measuring the free fraction of cyclosporine in plasma by equilibrium dialysis. J Pharm Pharmacol 1987; 39: 384–5
Akhlaghi F, Keogh AM, Brown KF. Unbound cyclosporine and allograft rejection after heart transplantation. Transplantation 1999; 67: 54–9
Rowland M, Tozer TN. Clinical pharmacokinetics, concepts and applications. 3rd ed. Philadelphia: Williams & Wilkins, 1995
Legg B, Gupta SK, Rowland M. A model to account for the variation in cyclosporin binding to plasma lipids in transplant patients. Ther Drug Monit 1988; 10: 20–7
Urien S, Zini R, Lemaire M, et al. Assessment of cyclosporine A interactions with human plasma lipoproteins in vitro and in vivo in the rat. J Pharmacol Exp Ther 1990; 253: 305–9
Akhlaghi F, Ashley JJ, Keogh AM, et al. Indirect estimation of the unbound fraction of cyclosporine in plasma. Ther Drug Monit 1998; 20: 301–8
Shah AK, Brundage RC, Lake KD, et al. The estimation of the plasma free fraction of cyclosporine in rabbits and heart transplant patients: the application of a physiological model of renal clearance. Biopharm Drug Dispos 1995; 16: 59–70
Fois R. Binding of cyclosporine to plasma lipoproteins [PhD dissertation]. Sydney: University of Sydney, 1991
Sheiner LB, Beal SL. Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm 1981; 9: 503–13
Shagel L, Yu ABC. Applied biopharmaceutics and pharmacokinetics. 4th ed. Stamford: Appleton & Lange, 1999
Lemaire M, Pardridge WM, Chaudhuri G. Influence of blood components on the tissue uptake indices of cyclosporin in rats. J Pharmacol Exp Ther 1988; 244: 740–3
Rifai N, Chao F-F, Pham Q, et al. The role of lipoproteins in the transport and uptake of cyclosporin and dihydro-tacrolimus into HepG2 and JURKAT cell lines. Clin Biochem 1996; 29: 149–55
Prueksaritanont T, Koike M, Hoener B-A, et al. Transport and metabolism of cyclosporine in isolated rat hepatocytes, the effect of lipids. Biochem Pharmacol 1998; 43: 1997–2006
Prueksaritanont T, Hoener BA, Benet LZ. Effects of low-density lipoprotein and ethinyl estradiol on cyclosporine metabolism in isolated rat liver perfusions. Drug Metab Dispos 1992; 20: 547–52
Strong ML, Ueda CT. Effects of low and high density lipoproteins on renal cyclosporine A and cyclosporine G disposition in the isolated perfused rat kidney. Pharm Res 1997; 14: 1466–71
Venkataramanan R, Ptachcinski RJ, Burckart GJ, et al. Extraction ratio of cyclosporine in a liver transplant patient with organ rejection. J Pharm Sci 1985; 74: 901–2
Yee GC, Lennon TP, Gmur DJ, et al. Effect of age on cyclosporin pharmacokinetics in marrow transplant recipients. Transplant Proc 1987; 19: 1704–5
Lithell H, Odlind B, Selinus I, et al. Is the plasma lipoprotein pattern of importance for treatment with cyclosporine? Transplant Proc 1986; 1: 50–1
Brunner LJ, Vadiei K, Luke DR. Cyclosporine disposition in the hyperlipidaemic rat model. Res Commun Chem Pathol Pharmacol 1988; 59: 339–48
Awni WM, Heim-Duthoy K, Kasiske BL. Impact of lipoproteins on cyclosporine pharmacokinetics and biological activity in transplant patients. Transplant Proc 1990; 22: 1193–6
Bastani B, Ritterhouse M, Garvin P. Reduced nephrotoxicity of cyclosporine A in hypercholesterolemic cadaveric renal transplant patients. Clin Transpl 1994; 8: 413–5
Jacqz-Aigrain E, Montes C, Brun C, et al. Cyclosporine pharmacokinetics in nephrotic and kidney-transplanted children. Eur J Clin Pharmacol 1994; 47: 61–5
Nemunaitis J, Deeg HJ, Yee GC. High cyclosporin levels after bone marrow transplantation associated with hypertriglyceridaemia. Lancet 1986; II(8509): 744–5
Verrill HL, Gigis RE, Easterling RE, et al. Distribution of cyclosporine in blood of a renal-transplant recipient with type V hyperlipoproteinemia. Clin Chem 1987; 33: 423–8
deLorgeril M, Boissonnat P, Bizollon CA, et al. Pharmacokinetics of cyclosporine in hyperlipidaemic long-term survivors of heart transplantation: lack of interaction with the lipid-lowering agent, fenofibrate. Eur J Clin Pharmacol 1992; 43: 161–5
Akhlaghi F, Mclachlan AJ, Keogh AM, et al. Effect of simvastatin on cyclosporine unbound fraction and apparent blood clearance in heart transplant recipients. Br J Clin Pharmacol 1997; 44: 1–6
Lindholm A, Henricsson S. Intra- and interindividual variability in the free fraction of cyclosporine in plasma in recipients of renal transplants. Ther Drug Monit 1989; 11: 623–30
Billingham ME, Cary NRB, Hammond ME, et al. A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection: heart rejection study group. J Heart Transplant 1990; 9: 587–93
Rodl S, Fuchs G, Khoshsorur G, et al. Lipoprotein-induced modulation of cyclosporine-A-mediated immunosuppression. Eur J Clin Invest 1990; 20: 248–52
Stulnig TM, Klocker H, Harwood J, et al. In vivo LDL receptor and HMG-CoA reductase regulation in human lymphocytes and its alterations during ageing. Arterioscler Thromb Vasc Biol 1995; 15: 872–8
Luke DR. Immunosuppressive effect of cyclosporine in the hyperlipidemic rat model. Biopharm Drug Dispos 1992; 13: 635–45
Kobashigawa JA, Kasiske BL. Hyperlipidaemia in solid organ transplantation. Transplantation 1997; 63: 331–8
Keogh AM. Coronary artery disease in cardiac transplant recipients. Med J Aust 1995; 163: 212–4
Black IW, Wilcken DEL. Decreases in apolipoprotein(a) after renal transplantation: implications for lipoprotein(a) metabolism. Clin Chem 1992; 38: 353–7
Ingulli E, Tejani A. Severe hypercholesterolemia inhibits cyclosporin A efficacy in a dose-dependent manner in children with nephrotic syndrome. J Am Soc Nephrol 1992; 3: 254–9
Dimeny E, Tufveson G, Lithell H, et al. The influence of pretransplant lipoprotein abnormalities on the early results of renal transplantation. Eur J Clin Invest 1993; 23: 572–9
Dimeny E, Wahlberg J, Lithell H, et al. Hyperlipidaemia in renal transplantation: risk factor for long-term graft outcome. Eur J Clin Invest 1995; 25: 574–83
Massy ZA, Guijarro C, Wiederkehr MR, et al. Chronic renal allograft rejection: immunologic and nonimmunologic risk factors. Kidney Int 1996; 49: 518–24
Massy ZA, Kasiske L, Guijarro C. Clinical correlation between renal allograft failure and hyperlipidemia. Kidney Int 1995; 48: S56–9
Tan KKC, Trull AK, Utteridge JA, et al. Effect of dietary fat on the pharmacokinetics and pharmacodynamics of cyclosporine in kidney transplant recipients. Clin Pharmacol Ther 1995; 13: 425–33
Arnadottir M, Berg AL. Treatment of hyperlipidemia in renal transplant recipients. Transplantation 1997; 63: 339–45
Knopp RH. Drug treatment of lipid disorders. N Engl J Med 1999; 341: 498–511
Katznelson S, Wilkinson AH, Kobashigawa JA, et al. The effect of pravastatin on acute rejection after kidney transplantation: a pilot study. Transplantation 1996; 61: 1469–74
Goldberg R, Roth D. Evaluation of fluvastatin in the treatment of hypercholesterolemia in renal transplant recipients taking cyclosporine. Transplantation 1998; 62: 1559–64
Li PKT, Mak TWL, Chan TH, et al. Effect of fluvastatin on lipoprotein profiles in renal transplant patients with dyslipoproteinemia [abstract]. Kidney Int 1996; 50: 1409
Imagawa DK, Dawson S, Holt CD, et al. Hyperlipidaemia after liver transplantation. Transplantation 1996; 62: 934–42
Kobashigawa JA, Katznelson S, Laks H, et al. Effect of pravastatin on outcomes after cardiac transplantation. N Engl J Med 1995; 333: 621–7
Wanner C, Kramer-Guth A, Galle J. Use of HMG-CoA reductase inhibitors after kidney and heart transplantation: lipid lowering and immunosuppressive effects. BioDrugs 1997; 8: 387–93
Maggard MA, Ke B, Wang T, et al. Effects of pravastatin on chronic rejection of rat cardiac allografts. Transplantation 1998; 65: 149–55
White CM. Pharmacological effects of HMG CoA reductase inhibitors other than lipoprotein modulation. J Clin Pharmacol 1999; 39: 111–8
Katznelson S, Kobashigawa JA. Dual roles of HMG-CoA reductase inhibitors in solid organ transplantation: lipid lowering and immunosuppression. Kidney Int 1995; 48: S112–5
de Groen PC, Aksamit AJ, Rakela J, et al. Central nervous system toxicity after liver transplantation. The role of cyclosporine and cholesterol. N Engl J Med 1987; 317: 861–6
de Groen PC. Cyclosporine, low-density lipoprotein, and cholesterol. Mayo Clin Proc 1988; 63: 1012–21
Keown P, Kahan BD, Johnston A, et al. Optimization of cyclosporine therapy with new therapeutic drug monitoring strategies: report from the International Neoral TDM Advisory Consensus Meeting (Vancouver, November 1997). Transplant Proc 1998; 30: 1645–9
Amante AJ, Kahan BD. Abbreviated AUC strategy for monitoring cyclosporine microemulsion therapy in the immediate posttransplant period. Transplant Proc 1996; 28: 2162–3
Lindholm A. Therapeutic monitoring of cyclosporin: an update. J Clin Pharmacol 1990; 41: 273–83
Best NG, Tan KKC, Trull AK, et al. Pharmacodynamics of cyclosporine in heart and heart-lung transplant recipients. II: blood cyclosporine concentrations and other risk factors for lung allograft rejection. Transplantation 1996; 62: 1436–41
El Gamel A, Keevil B, Rahman A, et al. Cardiac allograft rejection: do trough cyclosporine levels correlate with the grade of histologic rejection? J Heart Lung Transplant 1997; 16: 268–74
Best NG, Trull AK, Tan KKC, et al. Pharmacodynamics of cyclosporine in heart and heart-lung transplant recipients. I: blood cyclosporine concentrations and other risk factors for cardiac allograftrejection. Transplantation 1996; 62: 1429–35
Nohria A, Ehtisham J, Ramahi TM. Optimum maintenance trough levels of cyclosporine in heart transplant recipients given corticosteroid-free regimen. J Heart Lung Transplant 1998; 17: 849–53
Mahalati K, Belitsky P, Sketris I, et al. Neoral monitoring by simplified sparse sampling area under the concentration-time curve: its relationship to acute rejection and cyclosporine nephrotoxicity early after kidney transplantation. Transplantation 1999; 68: 55–62
Belitsky P, Dunn S, Johnston A, et al. Impact of absorption profiling on efficacy and safety of cyclosporin therapy in transplant recipients. Clin Pharmacokinet 2000; 39: 117–25
Cantarovich M, Elstein E, de Varennes B, et al. Clinical benefit of Neoral dose monitoring with cyclosporine 2-hr post- dose levels compared with trough levels in stable heart transplant patients. Transplantation 1999; 68: 1839–42
Chapel H, Haeney M, Misbah S, et al. Transplantation. 4th ed. London: Blackwell Science, 1999
Katayama N, Houjou T, Takada K. Lymphatic transport of cyclosporin A from the abdominal cavity. Int J Pharmacol 1994; 110: 155–60
Takada K, Yoshimura N, Yoshikawa H, et al. Enhanced selective lymphatic delivery of cyclosporin A by solubilizers and intensified immunosuppressive activity against mice skin allograft. Pharm Res 1986; 3: 48–51
Acknowledgements
No sources of funding were used to assist in the preparation of this manuscript. There are no potential conflicts of interest directly relevant to to the contents of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Akhlaghi, F., Trull, A.K. Distribution of Cyclosporin in Organ Transplant Recipients. Clin Pharmacokinet 41, 615–637 (2002). https://doi.org/10.2165/00003088-200241090-00001
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-200241090-00001