Summary
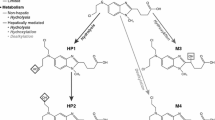
This article reviews the metabolism and pharmacokinetics of ifosfamide and their implications for the cytostatic efficacy and toxicity pattern of this alkylating agent. Ifosfamide is a prodrug that requires biotransformation to become cytotoxic. It is a structural isomer of cyclophosphamide from which it differs only in having the chlorethyl functions on different nitrogen atoms. This causes a considerable change in initial metabolism, although overall metabolism remains the same. Beside the formation of 4-hydroxy-ifosfamide (‘activated ifosfamide’), a second pathway with liberation of chloroacetaldehyde exists. Therefore, less activated drug is formed than during cyclophosphamide metabolism. This fact may well explain why higher doses of ifosfamide are required during treatment.
Chloroacetaldehyde may account for the adverse effects and therapeutic effects of the parent drug. This metabolite has been associated with central nervous system toxicity during ifosfamide treatment and was shown to deplete intracellular glutathione concentrations. Glutathione depletion may support the activity of alkylating metabolites in tumour cells, thus overcoming the relative resistance of the cells to alkylating agents. Possibly, this mechanism explains the lack of complete cross-resistance between ifosfamide and cyclophosphamide as well as the greater antitumour activity of ifosfamide in some tumours.
Urotoxicity of ifosfamide, which was the dose-limiting adverse effect, can be successfully attenuated by the use of mesna. Distinct pharmacokinetic properties of mesna are responsible for the fact that in contrast to other sulphydryl compounds the uroprotective activity of mesna does not imply a loss of therapeutic efficacy.
Similar content being viewed by others
References
Alarcon RA. Fluorometric determination of acrolein and related compounds with m-aminophenol. Analytical Chemistry 40: 1704–1708, 1968
Allen LM, Creaven PJ, Nelson RL. Studies on the human pharmacokinetics of isophosphamide (NSC-109724). Cancer Treatment Report 60: 451–458, 1976
Araujo CE, Tessler J. Treatment of ifosfamide-induced urothelial toxicity by oral administration of sodium 2-mercaptoethane sulphonate (mesna) to patients with inoperable lung cancer. European Journal of Cancer and Clinical Oncology 19: 195–201, 1983
Arndt CAS, Balis FM, McCully CL, et al. Cerebrospinal fluid penetration of active metabolites of cyclophosphamide and ifosfamide in rhesus monkeys. Cancer Research 48: 2113–2115, 1988
Bagley CM, Bostik FW, DeVita VT. Clinical pharmacology of cyclophosphamide. Cancer Research 33: 226–233, 1973
Ball Cr, Connors TA, Double JA, et al. Comparison of nitrogen-mustard-sensitive and — resistant Yoshida sarcomas. British Journal of Cancer 1: 319–327, 1966
Bielicki L, Voelcker G, Hohorst HJ. Enzymatic toxicogenation of ‘activated’ cyclophosphamide by 3′-5′ exonucleases. Journal of Cancer Research and Clinical Oncology 105: 27–29, 1983
Bierbaum W, Bremer K, Firusian N, et al. Chemotherapy in advanced sarcomas [in German]. Deutsche Medizinische Wochenschrift 106: 1181–1185, 1981
Boddy AV, Yule SM, Wyllie R, et al. Pharmacokinetics and metabolism of ifosfamide administered as a continuous infusion in children. Cancer Research 53: 3758–3764, 1993
Boos J, Welslau W, Ritter J, et al. Ifosfamide and its side-chain oxidized metabolites-urinary excretion under different pediatric treatment schedules. Klinische Padiatrie 204: 299–305, 1992
Brade WP, Herdrich K, Varini M. Ifosfamide — pharmacology, safety and therapeutic potential. Cancer Treatment Reviews 12: 1–47, 1985
Bramwell VHC, Mouridsen HT, Santoro, et al. Cyclophosphamide versus ifosfamide. Final report of a randomized phase II trial in adult soft tissue sarcoma. European Journal of Cancer and Clinical Oncology 23: 311–321, 1987
Brandt EL, Griffin AC. Reduction of toxicity of nitrogen mustards by cysteine. Cancer 4: 1030–1035, 1951
Brock N. The oxazaphosphorines. Cancer Treatment Reviews 10 (Suppl. A): 3–15, 1983
Brock N, Hilgard P, Pohl J, et al. Pharmacokinetics and mechanism of action of detoxifying low-molecular-weight thiols. Journal of Cancer Research and Clinical Oncology 108: 87–97, 1984
Brock N, Hohorst HJ. Metabolism of cyclophosphamide. Cancer 20: 900–904, 1967
Brock N, Pohl J. The development of mesna for regional detoxification. Cancer Treatment Reviews 10 (Suppl. A): 33–43, 1983
Brock N, Pohl J, Stekar J. Detoxification of urotoxic oxazaphosphorines by sulfhydryl compounds. Journal of Cancer Research and Clinical Oncology 100: 31–20, 1981a
Brock N, Pohl J, Stekar J. Studies on the urotoxicity of oxazaphosphorine cytostatics and its prevention. 2. Comparative study on the uroprotective efficacy of thiols and other sulfur compounds. European Journal of Cancer and Clinical Oncology 17: 1155–1163, 1981b
Brock N, Pohl J, Stekar J, et al. Studies on the urotoxicity of oxazaphosphorine cytostatics and its prevention-III. Profile of action of sodium 2-mercaptoethane sulfonate (mesna). European Journal of Cancer and Clinical Oncology 18: 1377–1387, 1982
Brock N, Stekar J, Pohl J, et al. Acrolein, the causative factor of urotoxic side-effects of cyclophosphamide, ifosfamide, trofosfamide and sufosfamide. Arzneimittel-Forschung 29: 659–661, 1979
Bryant BM, Jarman M, Baker MH, et al. Quantification by gas chromatography of N,N-di(3-chlorethyl) phospharamidic and (isophosphoramide mustard) in the plasma of patients receiving isophosphamide. Cancer Research 40: 4734–4738, 1980a
Bryant BM, Jarman M, Ford HT, et al. Prevention of isophosphamide-induced uroethelial toxicity with 2-mercaptoethane sulphonate sodium (mesna) in patients with advanced carcinoma. Lancet 2: 657–659, 1980b
Burk CD, Restiano I, Kaplan BS, et al. Ifosfamide-induced renal tubular dysfunction and rickets in children with Wilms tumor. Journal of Pediatrics 117: 331–335, 1990
Burns JJ, Conney A. Enzyme stimulation and inhibition in the metabolism of drugs. Proceedings of the Royal Society of Medicine 58: 955–960, 1965
Burton LC, James CA. Rapid method for the determination of ifosfamide and cyclophosphamide in plasma by high-performance liquid chromatography with solid-phase extraction. Journal of Chromatography 431: 450–454, 1988
Cerny T, Küpfer A. Stabilization and quantitative determination of the neurotoxic metabolite chloroacetaldehyde in the plasma of ifosfamide treated patients. Proceedings of the Fifth European Conference on Clinical Oncology 5, London, 1989, P0147, 1989
Cerny T, Kuepfer A, Zeugin T, et al. Bioavailability of subcutaneous ifosfamide and feasibility of continuous outpatient application in cancer patients. Annals of Oncology 1: 365–368, 1990
Cerny T, Lind M, Thatcher N, et al. A simple outpatient treatment with oral ifosfamide and oral etoposide for patients with small cell lung cancer (SCLC). British Journal of Cancer 60: 258–261, 1989
Cerny T, Margison M, Thatcher N, et al. Bioavailability of ifosfamide in patients with bronchial carcinoma. Cancer Chemotherapy and Pharmacology 18: 261–264, 1986
Colvin M. The comparative pharmacology of cyclophosphamide and ifosfamide. Seminars in Oncology 9 (Suppl. 1): 2–7, 1982
Connors TA. Protection against the toxicity of alkylating agents by thiols: the mechanism of protection and its relevance to cancer chemotherapy. European Journal of Cancer 2: 293–305, 1966
Connors TA, Cox PJ, Farmer PB, et al. Some studies of the active intermediates formed in the microsomal metabolism of cyclophosphamide and isophosphamide. Biochemical Pharmacology 23: 115–129, 1974
Cox PJ. Cyclophosphamide cystitis, identification of acrolein as the causative agent. Biochemical Pharmacology 28: 2045–2049, 1979
D’Incalci M, Bolis G, Facchinetti T, et al. Decreased half life of cyclophosphamide in patients under continual treatment. European Journal of Cancer 15: 7–10, 1979
Delepine N, Taillard F, Desbois JG, et al. CNS-side effects induced by ifosfamide-mesna in children with osteosarcoma. Biomedicine Pharmacotherapy 40: 173–175, 1986
Draeger U, Hohorst HJ. Permeation of cyclophosphamide (NSC-26271) metabolites into tumor cells. Cancer Treatment Report 60: 423–427, 1976
Draeger U, Peter G, Hohorst HJ. Deactivation of cyclophosphamide (NSC-26271) metabolites by sulfhydryl compounds. Cancer Treatment Report 60: 355–359, 1976
Duran M, Aarsen G, Fokkens RH, et al. 2-mercapto-etanesulfonate-cysteine disulfide excretion following the administration of 2-mercaptoethanesulfonate — a pitfall in the diagnosis of sulfite oxidase deficiency. Clinical Chimica Acta 111: 47–53, 1981
Elias AD, Eder JP, Shea T, et al. High dose ifosfamide with mesna uroprotection: a phase I study. Journal of Clinical Oncology 8: 170–178, 1990
Evans DAP, Mahgoub A, Sloan TP, et al. A family and population study of the genetic polymorphism of debrisoquine oxidation in a white British population. Journal of Medical Genetics 17: 102–105, 1980
Fraiser LH, Kanekal S, Kehrer JP. Cyclophosphamide toxicity: characterising and avoiding the problem. Drugs 42: 781–795, 1991
Friedman CM, Boger E. Colorimetric estimation of nitrogen mustard in aqueous media. Analytical Chemistry 33: 906–910, 1961
Furusawa S, Fujimura T, Sasaki K, et al. Potentiation of ifosfamide toxicity by chlordiazepoxide, diazepam and oxazepam. Chemical and Pharmaceutical Bulletin 37: 3420–3422, 1989
Gilard V, Malet-Martino MC, de Forni M, et al. Determination of the urinary excretion of ifosfamide and its phosphorated metabolites by phosphorus-31 nuclear magnetic resonance spectroscopy. Cancer Chemotherapy and Pharmacology 31: 387–394, 1993
Goldin A. Ifosfamide in experimental systems. Seminars in Oncology 9 (Suppl. I): 14–23, 1982
Goren MP. Determination of urinary 2- and 3-dechloroethylated metabolites of ifosfamide by high-performance liquid chromatography. Journal of Chromatography Biomedical Applications 570: 351–355, 1991
Goren MP. Oral mesna: a review. Seminars in Oncology 19 (Suppl. 12): 65–71, 1992
Goren MP, Pratt CB, Viar MJ. Tubular nephrotoxicity during long-term ifosfamide and mesna therapy. Cancer Chemotherapy and Pharmacology 25: 70–72, 1989
Goren MP, Wright RK, Pratt CB, et al. Dechloroethylation of ifosfamide and neurotoxicity. Lancet 2: 1219–1220, 1986
Goren MP, Wright RK, Pratt CB, et al. Potentiation of ifosfamide neurotoxicity, hematotoxicity, and tubular nephrotoxicity by prior cis-diamminedichloroplatinum(II) therapy. Cancer Research 47: 1457–1460, 1987
Graham MI, Shaw IC, Souhami RL, et al. Decreased half-life of cyclophosphamide during repeated high-dose administration. Cancer Chemotherapy and Pharmacology 10: 192–193, 1983
Grochow LB, Colvin M. Clinical pharmacokinetics of cyclophosphamide. In Ames et al. (Eds) Pharmacokinetics of anticancer agents in humans, pp. 135–154, Elsevier Science Publishers B. V., Amsterdam, 1983
Hergcbergs A, Brok-Simons F, Holtzman F. Erythrocyte glutathione and tumour response to chemotherapy. Lancet 339: 1074–1076, 1992
Hill DL, Laster Jr WF, Kirk MC, et al. Metabolism of ifosfamide and production of a toxic ifosfamide metabolite. Cancer Research 3: 1016–1022, 1973
Holdiness MR, Morgan LR. Electron capture gas chromatographic analysis of ifosfamide in human plasma and urine. Journal of Chromatography 275: 432–435, 1983
Holoye PY, Duelge RN, Hansen RM, et al. Prophylaxis of ifosfamide toxicity with oral acetylcysteine. Seminars in Oncology 10 (Suppl. 1): 66–71, 1983
Ikeuchi I, Amano T. Fluorometric determination of 4-hydroxyifos-famide in blood and urine. Chemical and Pharmaceutical Bulletin 33: 2416–2420, 1985
Ishikawa M, Takayanagi Y, Sasaki KI. Influence of buthione sulfoximine on the lethality of ifosfamide and ifosfamide-induced urotoxicity in mice. Research Communications in Chemical Pathology and Pharmacology 63: 455–458, 1989
James CA, Mant TGK, Rogers HJ. Pharmacokinetics of intravenous and oral sodium 2-mercaptoethane sulphonate (mesna) in normal subjects. British Journal of Clinical Pharmacology 23: 561–568, 1987
Jao JY, Jusko WJ, Cohen JL. Phenobarbital effects on cyclophosphamide pharmacokinetics in man. Cancer Research 32: 2761–2764, 1972
Jones MS, Murrell RD, Shaw IC. Excretion of sodium 2-mercaptoethanesulfonate (mesna) in the urine of volunteers after oral dosing. European Journal of Cancer and Clinical Oncology 21: 553–555, 1985
Juma FD, Rogers HJ, Trounce JR, et al. Pharmacokinetics of intravenous cyclophosphamide in man, estimated by gas-liquid chromatography. Cancer Chemotherapy and Pharmacology 1: 229–231, 1978
Kaijser GP, Beijnen JH, Bult A, et al. Gas chromatographic determination of ifosfamide in microvolumes of urine and plasma. Journal of Chromatography 571: 121–131, 1991
Kaijser GP, Beijnen JH, Jeunik EL, et al. Determination of chloroacetaldehyde, a metabolite of oxazaphosphorin cytostatic drugs, in plasma. Journal of Chromatography 614: 253–259, 1993
Kalow W. Genetic variation in the human hepatic cytochrome P-450 system. European Journal of Clinical Pharmacology 31: 633–641, 1987
Kellie SJ, Pritchard J, Bowman A, et al. Ifosfamide neurotoxicity in children. Journal of Clinical Oncology 5: 512–514, 1987
Klein OH, Wickramanyake PD, Christian E, et al. Therapeutic effects of single push or fractionated injections or continuous infusions of oxazaphosphorines (cyclophosphamide, ifosfamide, ASTAZ7557). Cancer 54 (Suppl. 6): 1193–1203, 1984
Kurowski V, Cerny T, Küpfer A, et al. Metabolism and pharmacokinetics of oral and intravenous ifosfamide. Journal of Cancer Research and Clinical Oncology 117 (Suppl. IV): 148–153, 1991
Kurowski V, Wagner T. Comparative pharmacokinetics of ifosfamide, 4-hydroxyifosfamide, chloroacetaldehyde, and 2- and 3-dechloroethylifosfamide in patients on fractionated intravenous ifosfamide therapy. Cancer Chemotherapy and Pharmacology 33: 36–42, 1993
Lambrechts H, Gheuens EOO, van Cauwenberghe KA, et al. Deter mination of ifosfamide by gas chromatography-mass spectrometry. Analytica Chimica Acta 247: 229–233, 1991
Lewis LD, Fitzgerald DL, Harper PG, et al. Fractionated ifosfamide therapy produces a time-dependent increase in ifosfamide metabolism. British Journal of Clinical Pharmacology 30: 725–732, 1990
Lewis LC, Meanwell CA. Ifosfamide pharmacokinetics and neurocity. Lancet 1: 175–176, 1990
Lind MJ, Margison JM, Cerny T, et al. Comparative pharmacokinetics and alkylating activity of fractionated intravenous and oral ifosfamide in patients with bronchogenic carcinoma. Cancer Research 49: 753–757, 1989a
Lind MJ, Margison JM, Cerny T, et al. Prolongation of ifosfamide elimination half-life in obese patients due to altered drug disposition. Cancer Chemotherapy and Pharmacology 25: 139–142, 1989b
Lind MJ, Margison JM, Cerny T, et al. The effect of age on the pharmacokinetics of ifosfamide. British Journal of Clinical Pharmacology 30: 140–143, 1990
Lind MJ, McGown AT, Hadfield JA, et al. The effect of ifosfamide and its metabolites on intracellular glutathione levels in vitro and in vivo. Biochemical Pharmacology 38: 1835–1840, 1989c
Lind MJ, Roberts HL, Thatcher N, et al. The effect of route of administration and fractionation of dose on the metabolism of ifosfamide. Cancer Chemotherapy and Pharmacology 23: 121–122, 1989d
Lokich J, Anderson N, Bern M, et al. Ifosfamide continuous infusion without mesna. Cancer 67: 883–885, 1991
Mahgoub A, Idle JR, Dring LG, et al. Polymorphic hydroxylation of debrisoquine in man. Lancet 2: 584–586, 1977
Manz I, Dietrich I, Przybylski M, et al. Identification and quantification of metabolite conjugates of activated cyclophosphamide and ifosfamide with mesna in urine by ion-pair extraction and fast atom bombardment mass spectrometry. Biomedical and Environmental Mass Spectrometry 12: 545–553, 1985
Margison JM, Wilkinson PM, Cerny T, et al. A simple quantitative HPLC assay for ifosfamide in biological fluids. Biomedical Chromatography 1: 101–103, 1986
Martino R, Crasnier F, Chouini-Lalanne N, et al. A new approach to the study of ifosfamide metabolism by the analysis of human body fluids with 31P nuclear magnetic resonance spectrometry. Journal of Pharmacology and Experimental Therapeutics 260: 1133–1144, 1992
McGown AT, Fox BW. A proposed mechanism of resistance to cyclophosphamide and phosphoramide mustard in a Yoshida cell line in vitro. Cancer Chemotherapy and Pharmacology 17: 223–236, 1986
Millar BC, Millar JL, Clutterbuck R, et al. Studies on the toxicity of cyclophosphamide in combination with mesna in vitro and in vivo. Cancer Treatment Reviews 10 (Suppl. A): 63–71, 1983
Moncrieff M, Foot A. Fanconi syndrome after ifosfamide, Cancer Chemotherapy and Pharmacology 23: 121–122, 1989
Morgan LR, Harrison EF, Hawke JE, et al. Toxicity of single vs fractionated-dose ifosfamide in non small cell lung cancer: a multi-center study. Seminars in Oncology 9 (Suppl. 1): 66–70, 1982
Mouridsen HT, Jacobsen E. Pharmacokinetics of cyclophosphamide in renal failure. Acta Pharmacologica et Toxicologica 36: 409–414, 1975
Munshi NC, Loehrer PJ, Williams SD, et al. Comparison of N-acetyl-cysteine and mesna as uroprotectors with ifosfamide combination chemotherapy in refractory germ cell tumors. Investigational New Drugs 10: 159–163, 1992
Nelson RL, Allen JM, Creaven PJ. Pharmacokinetics of divided doses of ifosfamide. Clinical Pharmacology and Therapeutics 19: 365–370, 1976
Norpoth K. Studies on the metabolism of isophosphamide (NSC-109724) in man. Cancer Treatment Report 60: 437–443, 1976
Norpoth K, Müller G, Raidt H. Isolation and characterisation of two main metabolites of ifosfamide from human urine. In German. Arzneimittel-Forschung 26: 1376–1377, 1976
Ormstadt K, Uehara N. Renal transport and disposition of Na-2-mercaptoethane sulfonate disulfide (dimesna) in the rat. Federation of European Biochemical Societies Letters 150: 354–358, 1982
Peter G, Wagner T, Hohorst HJ. Studies on 4-hydroperoxycyclophosphamide (NSC-181815): A simple preparation method and its application for the synthesis of a new class of ‘activated’ sulfur-containing cyclophosphamide (NSC-26271) derivatives. Cancer Treatment Report 60: 429–435, 1976
Philip PA, Lewis LD, James CA, et al. Ifosfamide plasma clearance in relation to polymorphic debrisoquine oxidation. Cancer Chemotherapy and Pharmacology 22: 321–324, 1988
Piazza E, Cattaneo MT, Varini M. Pharmacokinetic studies in lung cancer patients. Cancer 54: 1187–1192, 1984
Pohl J, Brock N, Stekar J. Toxicology, pharmacology, and interaction of sodium 2-mercaptoethane sulfonate (mesna). Current Chemotherapy 2: 1387–1389, 1981
Pratt CB, Green AA, Horowitz ME, et al. Central nervous system toxicity following the treatment of pediatric patients with ifosfamide/mesna. Journal of Clinical Oncology 4: 1253–1261, 1986
Radford JA, Margison JM, Swindell R, et al. The stability of ifosfamide in aqueous solution and its stability for 7-day infusion by ambulatory pump. Cancer Chemotherapy and Pharmacology 26: 144–146, 1990
Roberts HL, Lind MJ, Thatcher N, et al. Urinary ifosfamide metabolite profile after oral and intravenous ifosfamide. British Journal of Cancer 58: 262–265, 1988
Rodriquez V, McCredie KB, Keating MJ, et al. Ifosfamide therapy for hematological malignancies in patients refractory to prior treatment. Cancer Treatment Report 62: 493–497, 1978
Salloum E, Flamant F, Ghosn M, et al. Irreversible encephalopathy with ifosfamide/mesna. Journal of Clinical Oncology 5: 1303–1304, 1987
Sangster G, Kaye SB, Calman KC, et al. Failure of 2-mercaptoethane sulphonate sodium (Mesna) to protect against ifosfamide nephrotoxicity. European Journal of Cancer and Clinical Oncology 20: 435–436, 1984
Scheef W, Klein HO, Brock N, et al. Controlled clinical studies with an antidote against the urotoxicity of oxazaphosphorines: preliminary results. Cancer Treatment Report 63: 501–505, 1979
Scheulen ME, Niederle N, Bremer K, et al. Efficacy of ifosfamide in refractory malignant diseases and uroprotection by mesna: results of a clinical phase II study with 151 patients. Cancer Treatment Reviews 10 (Suppl. A): 93–101, 1983
Schuler U, Ehninger G, Wagner T. Repeated high-dose cyclophosphamide administration in bone marrow transplantation: exposure to activated metabolites. Cancer Chemotherapy and Pharmacology 20: 248–252, 1987
Shaw IC. Mesna and oxazaphosphorine cancer chemotherapy. Cancer Treatment Reviews 14: 359–364, 1987
Shaw IC, Graham MI, Jones M. The fate of sodium [U14C]-mercaptoethanesulfonate in the rat. Arzneimittel-Forschung 36: 487–489, 1986
Skinner R, Pearson ADJ, Price L, et al. Hypophosphatemic rickets after ifosfamide treatment in children. British Medical Journal 298: 1560–1561, 1989a
Skinner R, Pearson ADJ, Price L, et al. Nephrotoxicity in children. Lancet 2: 159, 1989b
Skinner R, Sharkey IM, Pearson AD, et al. Ifosfamide, mesna, and nephrotoxicity in children. Journal of Clinical Oncology 11: 173–190, 1993
Sladek NE, Doeden D, Powers JF, et al. Plasma concentration of 4-hydroxycyclophosphamide and phosphoramide mustard in patients repeatedly given high doses of cyclophosphamide in preparation for bone marrow transplantation. Cancer Treatment Report 68: 1247–1254, 1984
Sladek NE, Priest J, Doeden D, et al. Plasma half-life and urinary excretion of cyclophosphamide in children. Cancer Treatment Report 65: 1061–1066, 1980
Slavik MJ, Saiers JH. Phase I clinical study of acetylcysteine’s preventing ifosfamide-induced haematuria. Seminars in Oncology 10 (Suppl. 1): 62–65, 1983
Stofer-Vogel B, Cerny T, Borner M, et al. Oral bioavailability of mesna tablets. Cancer Chemotherapy and Pharmacology 32: 78–81, 1993a
Stofer-Vogel B, Cerny T, Kuepfer A, et al. Depletion of circulatingcyst(e)ine by oral and intravenous mesna. British Journal of Cancer 68: 590–593, 1993b
Talha MRZ, Rogers HJ. Rapid gas chromatographic determination of ifosfamide in biological fluids. Journal of Chromatography 311: 194–198, 1984
Voelcker G, Giera HP, Jäger L, et al. On the binding of cyclophosphamide and cyclophosphamide-metabolites to serum-albumin. In German. Zeitschrift Krebsforschuug 91: 127–142, 1978
Voelcker G, Haeglsperger R, Hohorst HJ. Fluorometric determination of activated cyclophosphamide and ifosfamide in blood. Journal of Cancer Research and Clinical Oncology 93: 233–240, 1979
Voelcker G, Jaschke A, Wrabetzz H, et al. Intracavitary high volume i.p. chemotherapy of S 180 ascites sarcoma in mice with 4-(S-ethanol)-sulfidocyclophosphamide in combination with protector thiols. Arzneimittel-Forschung 34: 1291–1298, 1984
Wagner T, Drings P. Pharmacokinetics and bioavailability of oral ifosfamide. Arzneimittel-Forschung 36: 878–880, 1986
Wagner T, Drings P. Pharmacokinetics and bioavailability of oral ifosfamide in tumour therapy. In Brade et al. (Eds) Ifosfamide in tumour therapy. Contributions to Oncology, Vol. 26, pp. 53–59, Karger, Basel, 1987
Wagner T, Ehninger G. Self-induction of cyclophosphamide and ifosfamide metabolism by repeated high-dose treatment. In Brade et al. (Eds) Ifosfamide in tumour therapy. Contributions to Oncology Vol. 26: pp. 69–75, Karger, Basel, 1987
Wagner T, Fenneberg K. Bioavailability of cyclophosphamide from oral formulations. European Journal of Clinical Pharmacology 26: 269–270, 1984a
Wagner T, Fenneberg K. Pharmacokinetics and bioavailability of cyclophosphamide from oral formulations. Arzneimittel-Forschung 34: 313–316, 1984b
Wagner T, Heydrich D, Jork T, et al. The influence of damaged liver parenchyma, renal insufficiency and hemodialysis on the pharmacokinetics of cyclophosphamide and its activated metabolites. In German. Arzneimittel-Forschung 30: 1588–1592, 1980
Wagner T, Heydrich D, Jork T, et al. Comparative study on human pharmacokinetics of activated ifosfamide and cyclophosphamide by a modified fluorometric test. Journal of Cancer Research and Clinical Oncology 100: 95–104, 1981
Wagner T, Mittendorff F, Walter E. Intracavitary chemotherapy with activated cyclophosphamide and simultaneous systemic detoxification with protector thiols in sarcoma 180 ascites tumor. Cancer Research 46: 2214–2219, 1986
Wagner T, Zink M, Schwieder G. Influence of mesna and cysteine on the systemic toxicity and therapeutic efficacy of activated cyclophosphamide. Journal of Cancer Research and Clinical Oncology 113: 160–165, 1987
Wang AL, Tew KD. Increased glutathione S-transferase activity in a cell line with acquired resistance to nitrogen mustards. Cancer Treatment Report 69: 677–682, 1985
Watkin SW, Husband DJ, Green JA, et al. Ifosfamide encephalopathy: a reappraisal. European Journal of Cancer and Clinical Oncology 25: 1303–1310, 1989
Wheeler BJ, Loehrer PJ, Williams SD, et al. Ifosfamide in refractory male germ cell tumors. Journal of Clinical Oncology 4: 28–34, 1986
Wiedemann GJ, Siemens HJ, Mentzel M, et al. Effects of temperature on the therapeutic efficacy and pharmacokinetics of ifosfamide. Cancer Research 53: 4268–4272, 1993
Wright KE, Garrod P, Shaw IC. Mechanism of enhanced cysteine excretion in urine during sodium 2-mercaptoethanesulfonate (mesna) administration. Human Toxicology 4: 546–550, 1985
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wagner, T. Ifosfamide Clinical Pharmacokinetics. Clin-Pharmacokinet 26, 439–456 (1994). https://doi.org/10.2165/00003088-199426060-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-199426060-00003