Abstract
As people grow old, their need for medications increases dramatically because of the higher incidence of chronic pain, diabetes mellitus, cardiovascular and neurological diseases in the elderly population. Furthermore, the elderly require special consideration with respect to drug delivery, drug interactions and adherence. In particular, patients with chronic neurological diseases often require multiple administration of drugs during the day to maintain constant plasma medication levels, which in turn increases the likelihood of poor adherence. Consequently, several attempts have been made to develop pharmacological preparations that can achieve a constant rate of drug delivery.
For example, transdermal lisuride and apomorphine have been shown to reduce motor fluctuations and duration of ‘off’ periods in advanced Parkinson’s disease, while rotigotine allows significant down-titration of levodopa without severe adverse effects. Thus, parkinsonian patients with long-term levodopa syndrome or motor disorders during sleep could benefit from use of transdermal lisuride and apomorphine. Moreover, transdermal dopaminergic drugs, particularly rotigotine, seem the ideal treatment for patients experiencing restless legs syndrome or periodic limb movement disorder during sleep, disorders that are quite common in elderly people or in association with neurodegenerative diseases.
Unlike dopaminergic drugs, transdermal treatments for the management of cognitive and behavioural dysfunction in patients with Parkinson’s disease and Alzheimer’s disease have inconsistent effects and no clearly established role. Nevertheless, because of their favourable pharmacological profile and bioavailability, the cholinesterase inhibitors tacrine and rivastigmine are expected to show at least the same benefits as oral formulations of these drugs, but with fewer severe adverse effects.
Transdermal delivery systems play an important role in the management of neuropathic pain. The transdermal lidocaine (lignocaine) patch is recommended as first-line therapy for the treatment of postherpetic neuralgia. Furthermore, in patients with severe persistent pain, transdermal delivery systems using the opioids fentanyl and buprenorphine are able to achieve satisfactory analgesia with good tolerability, comparable to the benefits seen with oral formulations.
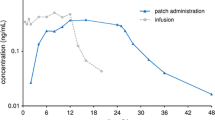
Transdermal administration is the ideal therapeutic approach for chronic neurological disorders in elderly people because it provides sustained therapeutic plasma levels of drugs, is simple to use, and may reduce systemic adverse effects. Several transdermal delivery systems are currently under investigation for the treatment of Parkinson’s disease, Alzheimer’s disease and neuropathic pain. Although most transdermal delivery systems treatments cannot be considered as first-line therapy at present, some of them provide clear advantages compared with other routes of administration and may become the preferred treatment in selected patients. In general, however, most transdermal treatments still require long-term evaluation in large patient groups in order to optimise dosages and evaluate the actual incidence of local and systemic adverse effects.



Similar content being viewed by others
References
Stahl SM, Wets KM. Recent advances in drug delivery technology for neurology. Clin Neuropharmacol 1988; 11: 1–17
Degim IT, Acarturk F, Erdogan D, et al. Transdermal administration of bromocriptine. Biol Pharm Bull 2003; 26: 501–5
Woitalla D, Muller T, Benz S, et al. Transdermal lisuride delivery in the treatment of Parkinson’s disease. J Neural Transm Suppl 2004; 68: 89–95
Benes H. Transdermal lisuride: short-term efficacy and tolerability study in patients with severe restless legs syndrome. Sleep Med 2006; 7: 31–5
Li GL, de Vries JJ, van Steeg TJ, et al. Transdermal iontophoretic delivery of apomorphine in patients improved by surfactant formulation pretreatment. J Control Release 2005; 101: 199–208
Priano L, Albani G, Brioschi A, et al. Transdermal apomorphine permeation from microemulsions: a new treatment in Parkinson’s disease. Mov Disord 2004; 19: 937–42
Priano L, Albani G, Brioschi A, et al. Nocturnal anomalous movement reduction and sleep microstructure analysis in parkinsonian patients during 1-night transdermal apomorphine treatment. Neurol Sci 2003; 24: 207–8
The Parkinson Study Group. Defining responder status in a clinical trial of the rotigotine transdermal system (SPM-962) in early Parkinson’s disease. Mov Disord 2001; 16: 981–2
Metman LV, Gillespie M, Farmer C, et al. Continuous transdermal dopaminergic stimulation in advanced Parkinson’s disease. Clin Neuropharmacol 2001; 24: 163–9
Poewe W, Luessi F. Clinical studies with transdermal rotigotine in early Parkinson’s disease. Neurology 2005; 65Suppl. 1: S11–4
Guldenpfennig WM, Poole KH, Sommerville KW, et al. Safety, tolerability, and efficacy of continuous transdermal dopaminergic stimulation with rotigotine patch in early-stage idiopathic Parkinson disease. Clin Neuropharmacol 2005; 28: 106–10
Pfeiffer RF. A promising new technology for Parkinson’s disease. Neurology 2005; Suppl. 1: S6–10
Stiasny-Kolster K, Kohnen R, Schollmayer E, et al., for the Rotigotine Sp 666 Study Group. Patch application of the dopamine agonist rotigotine to patients with moderate to advanced stages of restless legs syndrome: a double-blind, placebo-controlled pilot study. Mov Disord 2004; 19: 1432–8
Coelho F, Birks J. Physostigmine for Alzheimer’s disease. Cochrane Database Syst Rev 2001; (2): CD001499
Moller H-J, Hampel H, Hegerl U, et al. Double-blind, randomized, placebo-controlled clinical trial on the efficacy and tolerability of a physostigmine patch in patients with senile dementia of the Alzheimer type. Pharmacopsychiatry 1999; 32: 99–106
Sathyan G, Ritschel WA, Hussain AS. Transdermal delivery of tacrine: I. identification of a suitable delivery vehicle. Int J Pharm 1995; 114: 75–83
Jaskari T, Vuorio M, Kontturi K, et al. Controlled transdermal iontophoresis by ion-exchange fiber. J Control Release 2000; 67: 179–90
Tse FL, Laplanche R. Absorption, metabolism, and disposition of [14C] SDZ ENA 713, an acetylcholinesterase inhibitor, in minipigs following oral, intravenous, and dermal administration. Pharm Res 1998; 15: 1614–20
Muhlack S, Przuntek H, Muller T. Transdermal rivastigmine treatment does not worsen impaired performance of complex motions in patients with Alzheimer’s disease. Pharmacopsychiatry 2006; 39: 16–9
ClinicalTrials.gov. A service of the U.S. Institutes of Health developed by the National Library of Medicine [online]. Available from URL: http://clinicaltrials.gov/ct/showNCT00099242 [Accessed 2006 Jun 12]
Novartis. Clinical trials information [online]. Available from URL: http://www.novartisclinicaltrials.com/etrials/home.do [Accessed 2006 Jun 12]
Snaedal J, Johannesson T, Jonsson JE, et al. The effects of nicotine in dermal plaster on cognitive functions in patients with Alzheimer’s disease. Dementia 1996; 7: 47–52
White HK, Levin ED. Four-week nicotine skin patch treatment effects on cognitive performance in Alzheimer’s disease. Psychopharmacology 1999; 143: 158–65
Howe MN, Price IR. Effects of transdermal nicotine on learning, memory, verbal fluency, concentration, and general health in a healthy sample at risk for dementia. Int Psychogeriatr 2001; 13: 465–75
Kelton MC, Kahn HJ, Conrath CL, et al. The effects of nicotine on Parkinson’s disease. Brain Cogn 2000; 43: 274–82
Vieregge A, Sieberer M, Jacobs H, et al. Transdermal nicotine in PD: a randomized, double-blind, placebo-controlled study. Neurology 2001; 57: 1032–5
Ebersbach G, Stock M, Muller J, et al. Worsening of motor performance in patients with Parkinson’s disease following transdermal nicotine administration. Mov Disord 1999; 14: 1011–3
Lemay S, Chouinard S, Blanchet P, et al. Lack of efficacy of a nicotine transdermal treatment on motor and cognitive deficits in Parkinson’s disease. Prog Neuropsychopharmacol Biol Psychiatry 2004; 28: 31–9
Asthana S, Craft S, Baker LD, et al. Cognitive and neuroendocrine response to transdermal estrogen in postmenopausal women with Alzheimer’s disease: results of a placebo-controlled, double-blind, pilot study. Psychoneuroendocrinology 1999; 24: 657–77
Baker L, Sambamurti K, Craft S, et al. 17β-estradiol reduces plasma Aβ40 for HRT-naive postmenopausal women with Alzheimer disease: a preliminary study. Am J Geriatr Psychiatry 2003; 11: 239–44
Hall KA, Keks NA, O’Connor DW. Transdermal estrogen patches for aggressive behavior in male patients with dementia: a randomized, controlled trial. Int Psychogeriatr 2005; 17: 165–78
Davies PS, Galer BS. Review of lidocaine patch 5% studies in he treatment of postherpetic neuralgia. Drugs 2004; 64: 937–47
Chabal C, Russell LC, Burchiel KJ. The effect of intravenous lidocaine, tocainide, and mexiletine on spontaneously active fibers originating in rat sciatic neuromas. Pain 1989; 38: 333–8
Argoff CE, Galer BS, Jensen MP, et al. Effectiveness of the lidocaine patch 5% on pain qualities in three chronic pain states: assessment with the Neuropathic Pain Scale. Curr Med Res Opin 2004; 20: S21–8
Galer BS, Jensen MP, Ma T, et al. The lidocaine patch 5% effectively treats all neuropathic pain qualities: results of a randomized, double-blind, vehicle-controlled, 3-week efficacy study with use of the neuropathic pain scale. Clin J Pain 2002; 18: 297–301
Gammaitoni AR, Alvarez NA, Galer BS. Safety and tolerability of the lidocaine patch 5%, a targeted peripheral analgesic: a review of the literature. J Clin Pharmacol 2003; 43: 111–7
Galer BS, Gammaitoni AR. More than 7 years of consistent neuropathic pain relief in geriatric patients [letter]. Arch Intern Med 2003; 163: 628
Vranken JH, Dijkgraaf MG, Kruis MR, et al. Iontophoretic administration of S(+)-ketamine in patients with intractable central pain: a placebo-controlled trial. Pain 2005; 118: 224–31
Mystakidou K, Katsouda E, Tsilika E, et al. Transdermal therapeutic fentanyl-system (TTS-F). In Vivo 2004; 18: 633–42
Mystakidou K, Parpa E, Tsilika E, et al. Long-term management of noncancer pain with transdermal therapeutic system-fentanyl. J Pain 2003; 4: 298–306
Sittl R. Transdermal buprenorphine in the treatment of chronic pain. Expert Rev Neurother 2005; 5: 315–23
Likar R, Sittl R. Transdermal buprenorphine for treating noci ceptive and neuropathic pain: four case studies. Anesth Analg 2005; 100: 781–5
Olanow CW, Watts RL, Koller W. An algorithm (decision tree) for the management of Parkinson’s disease: treatment guidelines. Neurology 2001; 56: S1–88
Albanese A, Bonuccelli U, Brefel C, et al. Consensus statement on the role of acute dopaminergic challenge in Parkinson’s disease. Mov Disord 2001; 16: 197–201
van Laar T, van der Geest R, Danhof M, et al. Stepwise intravenous infusion of apomorphine to determine the therapeutic window in patients with Parkinson’s disease. Clin Neuropharmacol 1998; 2: 152–8
Nutt JG, Carter JH, Van Houten L, et al. Short- and long-duration responses to levodopa during the first year of levodopa therapy. Ann Neurol 1997; 42: 349–55
Hauser RA, Koller WC, Hubble JP, et al. Time course of loss of clinical benefit following withdrawal of levodopa/carbidopa and bromocriptine in early Parkinson’s disease. Mov Disord 2000; 15: 485–9
Hughes AJ, Frankel JP, Kempster PA, et al. Motor response to levodopa in patients with parkinsonian motor fluctuations: a follow-up study over three years. J Neurol Neurosurg Psychiatry 1994; 57: 430–4
Fahn S, Oakes D, Shoulson I, et al., for the Parkinson Study Group. Levodopa and the progression of Parkinson’s disease. N Engl J Med 2004; 351: 2498–508
Calon F, Grondin R, Morissette M, et al. Molecular basis of levodopa-induced dyskinesias. Ann Neurol 2000; 47: S70–8
Hardoff R, Sula M, Tamir A, et al. Gastric emptying time and gastric motility in patients with Parkinson’s disease. Mov Disord 2001; 16: 1041–7
Djaldetti R, Baron J, Ziv I, et al. Gastric emptying in Parkinson’s disease: patients with and without response fluctuations. Neurology 1996; 46: 1051–4
Pincus JH, Barry K. Protein redistribution diet restores motor function in patients with dopa-resistant ‘off’ periods. Neurology 1988; 38: 481–3
DeLong MR, Crutcher MD, Georgopoulos AP. Relations between movement and single cell discharge in the substantia nigra of the behaving monkey. J Neurosci 1983; 3: 1599–606
Nutt JG, Obeso JA, Stocchi F. Continuous dopamine-receptor stimulation in advanced Parkinson’s disease. Trends Neurosci 2000; 23: S109–15
Chase TN, Konitsiotis S, Oh JD. Striatal molecular mechanisms and motor dysfunction in Parkinson’s disease. Adv Neurol 2001; 86: 355–60
Soykan I, Sarosiek I, Shifflett J, et al. Effect of chronic oral domperidone therapy on gastrointestinal symptoms and gastric emptying in patients with Parkinson’s disease. Mov Disord 1997; 12: 952–7
Kurth MC, Adler CH. COMT inhibition: a new treatment strategy for Parkinson’s disease. Neurology 1998; 50: S3–14
Kurth MC, Adler CH, Hilaire MS, et al. Tolcapone improves motor function and reduces levodopa requirement in patients with Parkinson’s disease experiencing motor fluctuations: a multicenter, double-blind, randomized, placebo-controlled trial. Tolcapone Fluctuator Study Group I. Neurology 1997; 48: 81–7
Syed N, Murphy J, Zimmerman T, et al. Ten years’ experience with enteral levodopa infusions for motor fluctuations in Parkinson’s disease. Mov Disord 1998; 13: 336–8
Colzi A, Turner K, Lees AJ. Continuous subcutaneous waking day apomorphine in the long term treatment of levodopa induced interdose dyskinesias in Parkinson’s disease. J Neurol Neurosurg Psychiatry 1998; 64: 573–6
Stocchi F, Ruggieri S, Vacca L, et al. Prospective randomized trial of lisuride infusion versus oral levodopa in patients with Parkinson’s disease. Brain 2002; 125: 2058–66
Pfeiffer RF. Potential of transdermal drug delivery in Parkinson’s disease. Drugs Aging 2002; 19: 561–70
Sitruk-Ware R. Transdermal application of steroid hormones for contraception. J Steroid Biochem Mol Biol 1995; 53: 247–51
Berner B, John VA. Pharmacokinetic characterisation of transdermal delivery systems. Clin Pharmacokinet 1994; 26: 121–34
Martin GE, Williams M, Pettibone DJ, et al. Pharmacologic profile of a novel potent direct-acting dopamine agonist, (+)-4-propyl-9-hydroxynaphthoxazine [(+)-PHNO]. J Pharmacol Exp Ther 1984; 230: 569–76
Rupniak NMJ, Tye SJ, Jennings CA, et al. Antiparkinsonian efficacy of a novel transdermal delivery system (+)-PHNO in MPTP-treated squirrel monkeys. Neurology 1989; 39: 329–35
Coleman RJ, Lange KW, Quinn NP, et al. The antiparkinsonian actions and pharmacokinetics of (+)-4-propyl-9-hydroxynaphthoxazine (+PHNO): preliminary results. Mov Disord 1989; 4: 129–38
Ahlskog JE, Muenter MD, Bailey PA, et al. Parkinson’s disease monotherapy with controlled release MK-458 (PHNO): double-blind study and comparison to carbidopa/levodopa. Clin Neuropharmacol 1991; 14: 214–27
Smith LA, Jackson MG, Bonhomme C, et al. Transdermal administration of piribedil reverses MPTP induced motor deficits in the common marmoset. Clin Neuropharmacol 2000; 23: 133–42
Montestruct JL, Ziegler M, Rascol O, et al. A randomized, double-blind study of a skin patch of a dopamine agonist, piribedil, in Parkinson’s disease. Mov Disord 1999; 14: 336–41
MacMahon DG. Use of apomorphine in clinical practice. Adv Neurol 1999; 80: 529–33
Poewe W, Wenning GK. Apomorphine: an underutilized therapy for Parkinson’s disease. Mov Disord 2000; 15: 789–94
Gancher S. Pharmacokinetics of apomorphine in Parkinson’s disease. J Neural Transm Suppl 1995; 45: 137–41
Ondo W, Hunter C, Almaguer M, et al. A novel sublingual apomorphine treatment for patients with fluctuating Parkinson’s disease. Mov Disord 1999; 14: 664–8
van Laar T, Jansen EN, Essink AW, et al. Intranasal apomorphine in parkinsonian on-off fluctuations. Arch Neurol 1992; 49: 482–4
Hughes AJ, Bishop S, Lees AJ, et al. Rectal apomorphine in Parkinson’s disease [letter]. Lancet 1991; 337: 118
van Laar T, Jansen EN, Neef C, et al. Pharmacokinetics and clinical efficacy of rectal apomorphine in patients with Parkinson’s disease: a study of five different suppositories. Mov Disord 1995; 10: 433–9
Nyholm D. Pharmacokinetic optimisation in the treatment of Parkinson’s disease: an update. Clin Pharmacokinet 2006; 45: 109–36
Stocchi F, Berardelli A, Vacca L, et al. Apomorphine infusion and the long-duration response to levodopa in advanced Parkinson’s disease. Clin Neuropharmacol 2003; 26: 151–5
Colzi A, Turner K, Lees AJ. Continuous subcutaneous waking day apomorphine in the long term treatment of L-dopa induced interdose dyskinesias in Parkinson’s disease. J Neurol Neurosurg Psychiatry 1998; 64: 573–6
van der Geest R, Danhof M, Bodde HE. Iontophoretic delivery of apomorphine. I: in vitro optimization and validation. Pharm Res 1997; 14: 1798–803
van der Geest R, van Laar T, Gubbens-Stibbe JM, et al. Iontophoretic delivery of R-apomorphine. II: an in vivo study in patients with Parkinson’s disease. Pharm Res 1997; 14: 1804–10
van Laar T, van der Geest R, Danhof M. Future delivery systems for apomorphine in patients with Parkinson’s disease. In: Stern GM, editor. Parkinson’s disease. Advances in Neurology, Vol 80. Philadelphia (PA): Lippincott Williams and Wilkins, 1999: 535–44
Peira E, Scolari P, Gasco MR. Transdermal permeation of apomorphine through hairless mouse skin from microemulsions. Int J Pharmaceutics 2001; 226: 47–51
Cedarbaum JM. Clinical pharmacokinetics of anti-parkinsonian drugs. Clin Pharmacokinet 1987; 13: 141–78
Staal-Schreinemachers AL, Lakke JP. Bromocriptine long acting (LA) 50mg intramuscular (IM) for the on-off phenomenon in Parkinson’s disease [letter]. Acta Neurol Scand 1987; 75: 441
Vermesh M, Fossum GT, Kletzky OA. Vaginal bromocriptine: pharmacology and effect on serum prolactin in normal women. Obstet Gynecol 1988; 72: 693–8
Domino EF. Selective full dopamine D1-like (SKF-82958) and D2 like (N-0923) agonist combination in the MPTP monkey model of hemiparkinsonism. Brain Res Bull 1997; 43: 93–5
Belluzzi JD, Domino EF, May JM, et al. N-0923, a selective dopamine D2 receptor agonist, is efficacious in rat and monkey models of Parkinson’s disease. Mov Disord 1994; 9: 147–54
Happe S, Trenkwalder C. Role of dopamine receptor agonists in the treatment of restless legs syndrome. CNS Drugs 2004; 18: 27–36
Michaud M, Dumont M, Paquet J, et al. Circadian variation of the effects of immobility on symptoms of restless legs syndrome. Sleep 2005; 28(7): 843–6
Becker R, Giacobini E, Elble R, et al. Potential pharmacotherapy of Alzheimer disease: a comparison of various forms of physostigmine administration. Acta Neurol Scand Suppl 1988; 116: 19–32
Stern Y, Sano M, Mayeux R. Long-term administration of oral physostigmine in Alzheimer’s disease. Neurology 1988; 38: 1837–41.
Beller SA, Overall JE, Rhoades HM, et al. Long-term outpatient treatment of senile dementia with oral physostigmine. J Clin Psychiatry 1988; 49: 400–4
Gustafson L, Edvinsson L, Dahlgren N, et al. Intravenous physostigmine treatment of Alzheimer’s disease evaluated by psychometric testing, regional cerebral blood flow (rCBF) measurement, and EEG. Psychopharmacology (Berl) 1987; 93: 31–5
Thal LJ, Lasker B, Sharpless NS, et al. Plasma physostigmine concentrations after controlled-release oral administration [letter]. Arch Neurol 1989; 46: 13
Jenike MA, Albert MS, Heller H, et al. Oral physostigmine treatment for patients with presenile and senile dementia of the Alzheimer’s type: a double-blind placebo-controlled trial. J Clin Psychiatry 1990; 51: 3–7
Harrell LE, Jope RS, Falgout J, et al. Biological and neuropsychological characterization of physostigmine responders and nonresponders in Alzheimer’s disease. J Am Geriatr Soc 1990; 38: 113–22
Wagstaff AJ, McTavish D. Tacrine: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in Alzheimer’s disease. Drugs Aging 1994; 4: 510–40
Kankkunen T, Sulkava R, Vuorio M, et al. Transdermal iontophoresis of tacrine in vivo. Pharm Res 2002; 19: 705–8
Newhouse PA, Whitehouse PJ. Nicotinic-cholinergic systems in Alzheimer’s and Parkinson’s disease. In: Piasecki M, Newhouse PA, editors. Nicotine in psychiatry: psychopathology and emerging therapeutics. Washington, DC: American Psychiatric Press, 2000: 149–181
Sanberg PR, Silver AA, Shytle RD, et al. Nicotine for the treatment of Tourette’s syndrome. Pharmacol Ther 1997; 74: 21–5
Levin ED, Simon BB, Conners CK. Nicotine effects and attention-deficit/hyperactivity disorder. In: Piasecki M, Newhouse PA, editors. Nicotine in psychiatry: psychopathology and emerging therapeutics. Washington, DC: American Psychiatric Press, 2000: 203–214
Jones GM, Sahakian BJ, Levy R, et al. Effects of acute subcutaneous nicotine on attention, information processing and shortterm memory in Alzheimer’s disease. Psychopharmacology 1992; 108: 485–94
Rosin RA, Levine MD, Peskind E. Transdermal nicotine for agitation in dementia. Am J Geriatr Psychiatry 2001; 9: 443–4
Wilson AL, Langley LK, Monley J, et al. Nicotine patches in Alzheimer’s disease: pilot study on learning, memory, and safety. Pharmacol Biochem Behav 1995; 51: 509–14
Barros DM, Ramirez MR, Izquierdo I. Modulation of working, short- and long-term memory by nicotinic receptors in the basolateral amygdala in rats. Neurobiol Learn Mem 2005; 83: 113–8
May-Simera H, Levin ED. NMDA systems in the amygdala and piriform cortex and nicotinic effects on memory function. Brain Res Cogn Brain Res 2003; 17: 475–83
Levin ED, Tizabi Y, Rezvani AH, et al. Chronic nicotine and dizocilpine effects on regionally specific nicotinic and NMDA glutamate receptor binding. Brain Res 2005; 1041: 132–42
Buccafusco JJ, Letchworth SR, Bencherif M, et al. Long-lasting cognitive improvement with nicotinic receptor agonists: mechanisms of pharmacokinetic-pharmacodynamic discordance. Trends Pharmacol Sci 2005; 26: 352–60
Wurtman R, Blusztajn J, Maire J-C. ‘Autocannibalism’ of choline-containing membrane phospholipids in the pathogenesis of Alzheimer’s disease: a hypothesis. Neurochem Int 1985; 7: 369–72
Frederick B, Satlin A, Wald LL, et al. Brain proton magnetic resonance spectroscopy in Alzheimer disease: changes after treatment with xanomeline. Am J Geriatr Psychiatry 2002; 10: 81–8
Bodick NC, Offen WW, Shannon HE, et al. The selective muscarinic agonist xanomeline improves both the cognitive deficits and behavioral symptoms of Alzheimer disease. Alzheimer Dis Assoc Disord 1997; 11Suppl. 4: S16–22
Henderson V, Paganini-Hill A, Emanuel C, et al. Estrogen replacement therapy in older women: comparison between Alzheimer’s disease cases and nondemented control subjects. Arch Neurol 1994; 51: 896–900
Baldereschi M, Di-Carlo A, Lepore V, et al. Estrogen-replacement therapy and Alzheimer’s disease in the Italian Longitudinal Study on Aging. Neurology 1998; 50: 996–1002
McEwen B, Woolley C. Estradiol and progesterone regulate neuronal structure and synaptic connectivity in adult as well as developing brain. Exp Geront 1994; 29: 431–6
Xu H, Gouras GK, Greenfield JP, et al. Estrogen reduces neuronal generation of Alzheimer β-amyloid peptides. Nature Medicine 1998; 4: 447–51
Ancelin ML, Berr C. Hormonal replacement therapy and Alzheimer’s disease. All quiet on the western front? Psychol Neuropsychiatr Vieil 2003; 1: 251–7
Shneider L, Farlow M, Pogoda J. Potential role for estrogen replacement in the treatment of Alzheimer’s dementia. Am J Med 1997; 103: 46S–50S
Wang P, Liao S, Liu RS, et al. Effects of estrogen on cognition, mood, and cerebral blood flow in AD. Neurology 2000; 54: 2061–6
Mulnard RA, Cotman CW, Kawas C, et al. Estrogen replacement therapy for treatment of mild-to-moderate Alzheimer disease: a randomized controlled trial. JAMA 2000; 283: 1007–15
Henderson VW, Paganini-Hill A, Miller BL, et al. Estrogen for Alzheimer’s disease in women: randomized, double-blind, placebo-controlled trial. Neurology 2000; 54: 295–301
Parain K, Marchand V, Dumery B, et al. Nicotine, but not cotinine, partially protects dopaminergic neurons against MPTP-induced degeneration in mice. Brain Res 2001; 890: 347–50
Prasad C, Ikegami H, Shimizu I, et al. Chronic nicotine intake decelerates aging of nigrostriatal dopaminergic neurons. Life Sci 1994; 54: 1169–84
Wonnacott S, Kaiser S, Mogg A, et al. Presynaptic nicotinic receptors modulating dopamine release in the rat striatum. Eur J Pharmacol 2000; 393: 51–8
Rinne JO, Myllykyla T, Lonnberg P, et al. A postmortem study of brain nicotinic receptors in Parkinson’s and Alzheimer’s disease. Brain Res 1991; 547: 167–70
Shinotoh H, Namba H, Yamaguchi M, et al. In vivo mapping of brain cholinergic function in Parkinson’s disease and progressive supranuclear palsy. Adv Neurol 2001; 86: 249–55
Gammaitoni A, Gallagher RM, Welz-Bosna M. Topical ketamine gel: possible role in treating neuropathic pain. Pain Med 2000; 1: 97–100
Quan D, Wellish M, Gilden DH. Topical ketamine treatment of postherpetic neuralgia. Neurology 2003; 60: 1391–2
Acknowledgements
Dr Lorenzo Priano received a grant (3/2002) from Fondazione Italo Monzino Via Torquatto Tasso 14, Milano (Italy). The authors have no potential conflicts of interest that are directly relevant to the contents of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Priano, L., Gasco, M.R. & Mauro, A. Transdermal Treatment Options for Neurological Disorders. Drugs Aging 23, 357–375 (2006). https://doi.org/10.2165/00002512-200623050-00001
Published:
Issue Date:
DOI: https://doi.org/10.2165/00002512-200623050-00001