Abstract
The 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors specifically inhibit HMG-CoA reductase in the liver, thereby inhibiting the biosynthesis of cholesterol. These drugs significantly reduce plasma cholesterol level and long term treatment reduces morbidity and mortality associated with coronary heart disease.
The tolerability of these drugs during long term administration is an important issue. Adverse reactions involving skeletal muscle are not uncommon, and sometimes serious adverse reactions involving skeletal muscle such as myopathy and rhabdomyolysis may occur, requiring discontinuation of the drug. Occasionally, arthralgia, alone or in association with myalgia, has been reported.
In this article we review scientific data provided via Medline, adverse drug reaction case reports from the Swedish Drug Information System (SWEDIS) and the World Health Organization’s International Drug Information System (INTDIS) database, focusing on HMG-CoA reductase inhibitor-related musculoskeletal system events.
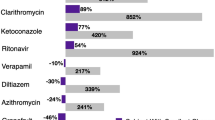
Cytochrome P450 (CYP) 3A4 is the main isoenzyme involved in the metabolic transformation of HMG-CoA reductase inhibitors. Individuals with both low hepatic and low gastrointestinal tract levels of CYP3A4 expression may be at in increased risk of myotoxicity due to potentially higher HMG-CoA reductase inhibitor plasma concentrations. The reported incidence of myotoxic reactions in patients treated with this drug class varies from 1 to 7% and varies between different agents. The risk of these serious adverse reactions is dose-dependent and may increase when HMG-CoA reductase inhibitors are prescribed concomitantly with drugs that inhibit their metabolism, such as itraconazole, cyclosporin, erythromycin and nefazodone. Electrolyte disturbances, infections, major trauma, hypoxia as well as drugs of abuse may increase the risk of myotoxicity. It is important that the potentially serious adverse reactions are recognised and correctly diagnosed so that the HMG-CoA reductase inhibitor may at once be withdrawn to prevent further muscular damage.









Similar content being viewed by others
References
Natio M, Hayashi T, Iguchi A. New approaches to the prevention of atherosclerosis. Drugs 1995; 50(3): 440–53
Frick MH, Elo O, Haapa K, et al. Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med 1987; 317(20): 1237–45
Menotti A, Blackburn H, Kromhout D, et al. Changes in population cholesterol levels and coronary heart disease deaths in seven countries. Eur Heart J 1997; 18(4): 566–71
Vogel RA. Coronary risk factors, endotelial function, and atherosclerosis: a review. Clin Cardiol 1997; 20: 426–32
Maher V, Sinfuego J, Chao P, et al. Primary prevention of coronary heart disease: what has WOSCOP told us and what questions remain? Drugs 1997; 54(1): 1–8
Hunninghake D. LDL-Cholesterol as a determinant of coronary heart disease. Clin Ther 1990; 12(5): 370–5
LaRosa JC, Hunninghake D, Bush D, et al. The cholesterol facts. A summary of the evidence relating dietary fats, serum cholesterol, and coronary heart disease. AHA Medical/Scientific Statement. Circulation 1990; 81(5): 1721–33
The lipid research clinics coronary primary prevention trial results I. Reduction in incidence of coronary heart disease. JAMA 1984; 251 (3): 351–64
Freedman JE, Loscalzo J. Endothelial dysfunction and atherothrombotic occlusive disease. Drugs 1997; 5Suppl. 3: 41–50
The lipid research clinics coronary primary prevention trial results. II. The relationship of reduction in incidence of coronary heart disease to cholesterol lowering. JAMA 1984; 251 (3): 365–74
Manninen V, Elo MO, Frick MH, et al. Lipid alterations and decline in the incidence of coronary heart disease in the Helsinki Heart Study. JAMA 1988; 260(5): 641–51
Nawrocki JW, Weiss SR, Davidson MH, et al. Reduction of LDL cholesterol by 25% to 60% in patients with primary hypercholesterolemia by atorvastatin, a new HMG-CoA reductase inhibitor. Arterioscler Thromb Vasc Biol 1995; 15(5): 678–82
The pravastatin multinational study group for cardiac risk patients. Effects of pravastatin in patients with serum total cholesterol levels from 5.2 to 7.8 mmol/liter (200 to 300 mg/dl) plus two additional atherosclerotic risk factors. The pravastatin multinational study group for cardiac risk patients. Am J Cardiol 1993; 72 (14): 1031–7
Bradford RH, Shear CL, Chremos AN, et al. Expanded clinical evaluation of lovastatin (EXCEL) study results. I. Efficacy in modifying plasma lipoproteins and adverse event profile in 8245 patients with moderate hypercholesterolemia. Arch Intern Med 1991; 151(1): 43–9
Zavoral JH, Haggerty BJ, Winick AG, et al. Efficacy of fluvastatin, a totally synthetic 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor. FLUENT Study Group. Fluvastatin long-term extension trial. Am J Cardiol 1995; 76(2): 37–40
Stein E, Sprecher D, Allenby KS, et al. Cerivastatin, a new potent synthetic HMG-Co-A reductase inhibitor: effect of 0,2 mg daily in subjects with primary hypercholesterolemia. J Cardiovasc Pharmacol Ther 1997; 2(1): 7–16
Lambrecht LJ, Malini PL. Efficacy and tolerability of simvastatin 20 mg vs pravastatin 20 mg in patients with primary hypercholesterolemia. European study group. Acta Cardiol 1993; 48(6): 541–54
Scandinavian simvastatin survival group. Randomized trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian simvastatin survival study (4S). Lancet 1994; 344: 1383–9
Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med 1996; 335(14): 1001–9
Pedersen TR, Kjekshus J, Pyorala K, et al. Effect of simvastatin on ischemic signs and symptoms in the Scandinavian simvastatin survival study (4S). Am J Cardiol 1998; 81(3): 333–5
Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland coronary prevention study group. N Engl J Med 1995; 333(20): 1301–7
Hebert PR, Gaziano JM, Chan KS, et al. Cholesterol lowering with statin drugs, risk of stroke and total mortality: an overview of randomized trials. JAMA 1997; 278(4): 313–21
Gordon DJ, Rifkind BM. High density lipoprotein: the clinical implications of recent studies. N Engl J Med 1989; 321(19): 1311–6
Grundy SM. Cholesterol metabolism in man (medical progress). West J Med 1978; 128: 13–25
Stubbs RJ, Schwartz M, Bayne WF. Determination of mevinolin and mevinolinic acid in plasma and bile by reversed-phase high-performance liquid chromatography. Philadelphia: Elsevier Science Publishers B.V., 1986; 383: 438–43
Alberts AW, Chen J, Kuron G, et al. Mevinolin: a highly potent competetive inhibitor of hydroxymetylglutaryl-coenzym A reductase and a cholesterol-lowering agent. Biochemistry 1980; 77(7): 3957–61
Pentikainen PJ, Saraheimo M, Schwartz JI, et al. Comparative pharmacokinetics of lovastatin, simvastatin and pravastatin in human. J Clin Pharmacol 1992; 32: 136–40
Lijnen P, Celis H, Desager JP, et al. Changes in plasma lipids, lipoproteins and apolipoproteins in hypercholesterolaemic patients treated with pravastatin. J Hum Hypertens 1995; 9(7): 557–64
Fukami M, Maeda N, Fukushige J, et al. Effects of HMG-CoA reductase inhibitors on skeletal muscles of rabbits. Res Exp Med Berl 1993; 193(5): 263–73
Garnett WR. A review of current clinical findings with fluvastatin. Am J Cardiol 1996; 78Suppl. 6A: 20–5
Levy RI, Troendle AJ, Fattu JM. Aquarter century of drug treatment of dyslipoproteinemia, with a focus on the new HMG-CoA reductase inhibitor fluvastatin. Circulation 1993; 87 Suppl. III: 45–53
Gibson DM, Bron NJ, Richens A, et al. Effect of age and gender on pharmacokinetics of atorvastatin in humans. J Clin Pharmacol 1996; 36: 242–6
Lea AP, McTavish D. Atorvastatin a review of its pharmacology and therapeutic potential in the management of hyperlipidaemias. Drugs 1997; 53(5): 828–47
Muck W, Ochmann K, Rohde G, et al. Influence of erythromycin pre- and co-treatment on single-dose pharmacokinetics of the HMG-CoA reductase inhibitor cerivastatin. Eur J Clin Pharmacol 1998; 53: 469–73
Peters TK, Mehra M, Muratti EN. Efficacy and safety of fluvastatin in hypertensiv patients, An analysis of a clincal trial database. Am J Hypertens 1993; 6: 340–5
Transon C, Leemann T, Dayer P. In vitro comparative inhibition profiles of major human drug metabolising cytochrome P450 isozymes (CYP2C9, CYP2D6 and CYP3A4) by HMG-CoA reductase inhibitors. Eur J Clin Pharmacol 1996; 50(3): 209–15
Gadbut AP, Caruso AP, Galper JB. Differential sensitivity of C2-C12 striated muscle cells to lovastatin and pravastatin. J Mol Cell Cardiol 1995; 27(10): 2397–402
Prueksaritanont T, Gorham LM, Ma B, et al. In vitro metabolism of simvastatin in humans [SBT] identification of metabolizing enzymes and effect of the drug on hepatic P450s. Drug Metab Dispos 1997; 25(10): 1191–9
Nordin C, Dahl ML, Eriksson M, et al. Is the cholesterol-lowering effect of simvastatin influenced by CYP2D6 polymorphism? [letter]. Lancet 1997; 350(9070): 29–30
Zhou LX, Finley DK, Hassell AE, et al. Pharmacokinetic interaction between isradipine and lovastatin in normal, female and male volunteers. J Pharmacol Exp Ther 1995; 273(1): 121–7
Chong PH, Seeger JD. Atorvastatin calcium: an addition to HMG-CoA reductase inhibitors. Pharmacotherapy 1997; 17(6): 1157–77
Wolfgan M. Rational assessment of the interaction profile of cerivastatin supports its low propensity for drug interaction. Drugs 1998; 56Suppl. 1: 15–23
Jönsson B, Johansson M, Kjekshus, et al. Cost-effectiveness of cholesterol lowering: result from the Scandinavian simvastatin survival study (4S). Eur Heart J 1996; 17: 1001–7
Caro J, Klittich W, McGuire A, et al. The west Scotland coronary prevention study: economic benefit analysis of primary prevention with pravastatin. BMJ 1997; 315: 1577–82
Steinhagen-Thiessen E. Comparative efficacy and tolerability of 5 and 10 mg simvastatin and 10 mg pravastatin inmoderate primary hypercholesterolemia. Simvastatin pravastatin European study group. Cardiology 1994; 85: 244–54
Bertolini S, Bon GB, Campbell LM, et al. Efficacy and safety of atorvastatin compared to pravastatin in patients with hypercholesterolemia. Atherosclerosis 1997: 130(1-2); 191–7
Bradford RH, Shear CL, Chremos AN, et al. Expanded clinical evaluation of lovastatin (EXCEL) study results: two-year efficacy and safety follow-up. Am J Cardiol 1994; 74(7): 667–73
Kantola T, Kivistö KT, Neuvonen PJ. Erythromycin and verapamil considerably increase serum simvastatin and simvastatin acid concentrations. Clin Pharmacol Ther 1998; 64(2): 177–82
Pichard L, Domergue J, Fourtanier G, et al. Metabolism of the new immunosuppressor cyclosporin G by human liver cytochromes P450. Biochem Pharmacol 1996; 51(5): 591–8
Arnadottir M, Eriksson LO, Thysell H, et al. Plasma concentration profiles of simvastatin 3-Hydroxy-3-Metyl-glutaryl-Coenzyme A reductase inhibitory activity in kidney transplant recipients with and without ciclosporin. Nephron 1993; 65: 410–3
Olbricht C, Wanner C, Eisenhauer T, et al. Accumulation of lovastatin, but not pravastatin, in the blood of cyclosporinetreated kidney graft patients after multiple doses. Clin Pharmacol Ther 1997; 62(3): 311–21
Neuvonen PJ, Kantola T, Kivistö KT. Simvastatin but not pravastatin is very susceptible to interaction with the CYP3A4 inhibitor itraconazole. Clin Pharmacol Ther 1998; 63(3): 332–41
Kantola T, Kivistö KT, Neuvonen PJ. Grapefruit juice greatly increases serumconcentrations of lovastatin and lovastatin acid. Clin Pharmacol Ther 1998; 63(4): 397–402
Lilja JJ, Kvistö KT, Neuvonen PJ. Grapefruit juice-simvastatin interaction: effect on concentrations of simvastatin, simvastatin acid and HMG-CoA reductase inhibitors. Clin Pharmacol Ther 1998; 64(5): 477–83
Langer T, Levy RI. Acute muscular syndrome associated with administration of clofibrate. N Engl J Med 1968; 279(16): 856–8
Bank WJ, Dimauro S, Bonilla E, et al. Adisorder of muscle lipid metabolsm and myoglobinuria: absence of carnitine palmityl transferase. N Engl J Med 1975; 292: 443–9
Chu PH, Chen WJ, Chiang CW, et al. Rhabdomyolysis, acute renal failure and hepatopathy induced by lovastatin monotherapy. JPN Heart J 1996; 38(4): 541–5
Grundy SM. HMG-CoA reductase inhibitors for treatment of hypercholesterolemia. N Engl J Med 1988; 319: 24–32
Davidson M, McKenney J, Stein E, et al. Comparison of one year efficacy and safety of atorvastatin versus lovastatin in primary hypercholesterolemia. Am J Cardiol 1997; 79: 1475–81
Gebhard RL, Ewing SL, Schlasner LA, et al. Effect of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibition on human gut mucosa. Lipids 1991; 26(7): 492–4
Lane RJM, Mastaglia FL. Drug induced myopathies in man. Lancet 1978; II(8089): 562–6
Fichtl B, Kurz H. Binding of drugs to human muscle. Eur J Clin Pharmacol 1978; 14: 335–40
Kantola T, Kvistö KT, Neuvonen PJ. Effect of itraconazole on the pharmacokinetics of atorvastatin. Clin Pharmacol Ther 1998; 64: 58–65
Giordano N, Senesi M, Mattii G, et al. Polymyositis associated with simvastatin. Lancet 1997; 349: 1600–1
Schalke BB, Schmitdt B, Toyka K, et al. Pravastatin-associated inflammatorymyopathy. N Engl J Med 1992; 327(9): 649–50
Tobert JA. Efficacy and long term adverse effect pattern of lovastatin. Am J Cardiol 1988: 62; 28–34
Nakahara K, Kuriyama M, Yoshidome H, et al. Experimental simvastatin-induced myopathy in rabbits. J Neurol Sci 1992; 113(1): 114–7
Hino I, Akama H, Furuya T, et al. Pravastatin-induced rhabdomyolysis in a patient with mixed connective tissue disease. Arthritis-Rheum 1996: 39(7); 1259–60
Kogan AD, Orensterin S. Lovastatin-induced acute rhabdomyolysis. Postgrad Med J 1990; 66: 293–6
Prendergast BD, George CF. Drug-induced rhabdomyolysis mechanisms and management. Postgrad Med J 1993; 69(811): 333–6
Hanna JP, Ramundo ML. Rhabdomyolysis and hypoxia associated with prolonged propofol infusion in children. Neurology 1998; 50: 301–3
Phan T, McLeod JG, Pollard JD, et al. Peripheral neuropathy associated with simvastatin. J Neurol, Neurosurg Psychiatry 1995; 58(5): 625–8
Jacobs MB. HMG-CoA reductase inhibitor therapy and peripheral neuropathy. Ann Intern Med 1994: 120(11); 970
Dart A, Jerums G, Nicholson G, et al. A multicenter, doubleblind, one-year study comparing safety and efficacy of atorvastatin versus simvastatin in patients with hypercholesterolemia. Am J Cardiol 1997: 80(1); 39–44
Jokubaitis LA. Updated clinical safety experience with fluvastatin. Am J Cardiol 1994: 73(14); 18–24
Dujovne CA, Chremos AN, Pool JL, et al. Expanded clinical evaluation of lovastatin (EXCEL) study results: IV. Additional perspectives on the tolerability of lovastatin. Am J Med 1991; 91(1B): 25–30
Miserez AR, Rossi FA, Keller U. Prediction of the therapeutic response to simvastatin by pretreatment lipid concentration in 2082 subjects. Eur J Clin Pharmacol 1994; 46(2): 107–14
Chariot P, Abadia R, Agnus D, et al. Simvastatin-induced rhabdomyolysis followed by a MELAS syndrome. Am J Med 1993: 94(1); 109–10
Rimon D, Ludatscher R, Cohen L. Clofibrate-induced muscular syndrome. Case report with ultrastructural findings and review of the literature. Isr J Med Sci 1984; 20(11): 1082–6
Gorriz JL, Sancho A, Lopez-Martin JM, et al. Rhabdomyolysis and acute renal failure associated with gemfibrozil therapy. Nephron 1996; 74(2): 437–8
Walsh JC, Stewart P, Boyd I, et al. Mitochondrial myopathy developing on treatment with the HMG CoAreductase inhibitorssimvastatin and pravastatin. Aust NZ J Med 1995; 25: 374–5
Goldman JA, Fishman AB, Lee JE, et al. The role of cholesterollowering agents in drug induced rhabdomyolysis and polymyositis. Arthritis Rheum 1989; 32: 358–9
Sonoda Y, Gotow T, Kuriyama M, et al. Electrical myotonia of rabbit skeletal muscles by HMG-CoA reductase inhibitors. Muscle Nerve 1994; 17(8): 891–7
Morita I, Sato I, Ma L, et al. Enhancement ofmembrane fluidity in cholesterol-poor endothelial cells pre-treated with simvastatin. Endothelium 1997; 5(2): 107–13
Levy Y, Leibowitz R, Aviram M, et al. Reduction of plasma cholesterol by lovastatin normalizes erythrocyte membrane fluidity in patients with severe hypercholesterolaemia. Br J Clin Pharmacol 1992; 34(5): 427–30
Hochgraf E, Levy Y, Aviram M, et al. Lovastatin decreases plasma and platelet cholesterol levels and normalizes elevated platelet fluidity and aggregation in hypercholesterolemic patients. Metabolism 1994; 43(1): 11–7
Lijnen P, Celis H, Fagard R, et al. Influence of cholesterol lowering on plasma membrane lipids and cationic transport systems. J-Hypertens 1994; 12(1): 59–64
Davidson MH, Stein EA, Dujovne CA, et al. The efficacy and six-week tolerability of simvastatin 80 and 160 mg/day. Am J Cardiol 1997; 79(1): 38–42
Smith PF, Eydelloth RS, Grossman SJ, et al. HMG-CoA reductase inhibitor-induced myopathy in the rat: cyclosporine A interaction and mechanism studies. J Pharmacol Exp Ther 1991; 257(3): 1225–35
Masters BA, Palmoski MJ, Flint OP, et al. In vitro myotoxicity of the 3-hydroxy-3-methylglutaryl coenzyme Areductase inhibitors, pravastatin, lovastatin, and simvastatin, using neonatal rat skeletal myocytes. Toxicol Appl Pharmacol 1995; 131(1): 163–74
Nakahara K, Yada T, Kuriyama M, et al. Cytosolic Ca2+ increase and cell damage in L6 rat myoblasts by HMG-CoA reductase inhibitors. Biochem Biophys Res Commun 1994; 202(3): 1579–85
Segaert MF, De-Soete C, Vandewiele I, et al. Drug-interactioninduced rhabdomyolysis. Nephrol Dial Transplant 1996; 11(9): 1846–7
Wu CY, Benet LZ, Hebert MF, et al. Differentiation of absorption and first-pass gut and hepatic metabolism in humans: studies with cyclosporine. Clin Pharmacol Ther 1995; 58(5): 492–7
Meier C, Stey C, Brack T, et al. Rhabdomyolyse bei mit simvastatin und ciclosporin behandelten patienten: rolle der aktivität des cytochrom-P450-Enzymsystems der leber. Schweiz Med Wochenschr 1995; 125: 1342–6
Lown KS, Mayo RR, Leichtman AB, et al. Role of intestinal P-glycoprotein (mdr1) in interpatient variation in the oral bioavailability of cyclosporine. Clin Pharmacol Ther 1997; 62(3): 248–60
Shimada T, Yamazaki H, Mimura M, et al. Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians. J Pharmacol Exp Ther 1994; 270(1): 414–23
Kivistö K, Bookjans G, Fromm MF, et al. Expression of CYP3A4, CYP3A5 and CYP3A7 in human duodenal tissue. Br J Clin Pharmacol 1996; 42: 387–9
Thummel KE, Shen DD, Podoll TD, et al. Use of midazolam as a human cytochrome P450 3A probe: II. characterization of inter- and intraindividual hepatic CYP3A variability after liver transplantation. J Pharmacol Exp Ther 1994; 271(1): 557–66
Kolars JC, Lown KS, Schmiedlin-Ren P, et al. CYP3A gene expression in human gut epithelium. Pharmacogenetics 1994; 4(5): 247–59
Pierce LR, Wysowski DK, Gross TP. Myopathy and rhabdomyolysis associated with lovastatin-gemfibrozil combination therapy. JAMA 1990; 264(1): 71–5
Duell PB, Connor WE, Illingworth DR. Rhabdomyolysis after taking atorvastatin with gemfibrozil. Am J Cardiol 1998; 81(3): 368–9
Van Puijenbroek EP, Du Buf-Vereijken PW, Spooren PF, et al. Possible increased risk of rhabdomyolysis during concomitant use of simvastatin and gemfibrozil. J Intern Med 1996; 240(6): 403–4
Tal A, Rajeshawari M, Isley W. Rhabdomyolysis associated with simvastatin-gemfibrozil therapy. South Med J 1997; 90(5): 546–7
Panucio V, Enia G, Parlongo S, et al. Severe rhabdomyolysis induced by a retard formulation of bezafibrate in a CAPD patient [letter]. Nephron 1996; 73: 736
Schmassmann-Suhijar D, Bullingham R, Gasser R, et al. Rhabdomyolysis due to interaction of simvastatin with mibefradil. Lancet 1998; 351: 1929–30
Horn M. Coadministration of itraconazole with hypolipidemic agens may induce Rhabdomyolysis in healthy indiviuals [letter]. Arch Dermatol 1996: 132; 1254
Jacobson RH, Wang P, Glueck CJ. Myositis and rhabdomyolysis associated with concurrent use of simvastatin and nefazodone [letter]. JAMA 1997: 277(4); 296–7
Imai Y, Watanabe N, Hashimoto J, et al. Muscle cramps and elevated serum creatine phosphokinase levels induced by Beta-adrenoreceptor blockers. Eur J Clin Pharmacol 1995; 48: 29–34
Korzets A, Ori Y, Floro S, et al. Severe hyponatremia after water intoxication: a potential cause of rhabdomyolysis. Am J Med Sci 1996; 12(2): 92–4
Melberg A, Holme E, Oldfors A, et al. Rhabdomyolysis in autosomal dominant progressive external ophthalmoplegia. Neurology 1998; 50(1): 299–300
Engel WK, Vick NA, Glueck CJ, et al. A skeletal-muscle disorder associated with intermittent symptoms and possible defect of lipid metabolism. N Engl J Med 1970; 282(13): 697–704
Carrascosa M, Pascual F, Borobio MV, et al. Rhabdomyolysis associated with acute Q fever. Clin Infect Dis 1997; 25(5): 1243–4
Hortobagyi T, Denahan T. Variability in creatine kinase: methodological, exercise, and clinically related factors. Int J Sports Med 1989; 10: 69–80
Thompson PD, Gadaleta PA, Yurgalevitch S, et al. Effects of exercise and lovastatin on serum creatine kinase activity. Metabolism 1991; 40(12): 1333–6
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ucar, M., Mjörndal, T. & Dahlqvist, R. HMG-CoA Reductase Inhibitors and Myotoxicity. Drug-Safety 22, 441–457 (2000). https://doi.org/10.2165/00002018-200022060-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00002018-200022060-00003