
Robotic surgical techniques for standard segmentectomies
Introduction
Anatomic pulmonary segmentectomy is the removal of a bronchopulmonary subdivision of lung parenchyma determined by its tertiary (segmental) bronchus and corresponding pulmonary arterial branch, often bordered by intersegmental veins. Pulmonary segmentectomies can be further subdivided as either standard (simple/typical) or complex (atypical). Standard segmentectomies include right and left lower lobe superior segmentectomies (S6), basilar segmentectomies (S7-10), left upper lobe trisegmentectomy (S1-3), and lingulectomy (S4-5) (1,2).
Indications for segmentectomy
Segmentectomies have been performed through thoracotomies for the management of bronchiectasis and tuberculosis for many years, though their application for curative resection of malignancy has been debated. In a randomized, prospective trial published by the Lung Cancer Study Group in 1995, patients with T1N0 non-small cell lung cancer (NSCLC) who underwent a limited sublobar resection had a three-fold increase in local recurrence rates and 50% increase in death with cancer compared to lobectomy patients (3). However, the trial included 40 non-anatomical wedge resections out of the 122 limited resection patients (32.8%) as well as tumors up to 3 cm in size on chest X-ray without the benefit of routine axial imaging or positron emission tomography (PET). Moreover, the initially reported higher rates of overall death and death with cancer among limited resection patients lost significance following updated results that were published in 1996 (3,4).
Within the last decade, there have been a number of retrospective studies that challenged the Lung Cancer Study Group’s findings. Meta-analyses by Cao et al. and Guo et al. included NSCLC patients who were intentionally selected for anatomic segmentectomy due to their early stage, favorable histopathology, and anatomic location, as opposed to a more biased selection of patients who were unable to physiologically tolerate lobectomy. Both meta-analyses demonstrated no differences in local or distant recurrence, overall survival, or recurrence-free survival in intentionally selected patients for segmentectomy as compared to lobectomy (5,6).
Pulmonary segmentectomies may be performed using open, thoracoscopic, and robotic approaches. The particular advantages of robotic-assisted segmentectomy have been well-described within the literature (7-11). In particular, the robotic platform affords the surgeon excellent three-dimensional visualization for the performance of an extensive nodal dissection which greatly helps in defining segmental anatomy. Additionally, given the variation inherently present in segmental vasculature, robotic surgery is particularly advantageous in performing segmentectomy as it permits flexibility in the operative approach. Decisions regarding fissure dissection, or the direction of approach, whether it be anterior to posterior similar to thoracoscopic surgery, or posterior to anterior, can be tailored to each patient’s particular anatomy. Moreover, defining intersegmental anatomy using near-infrared imaging with a fluorescing agent, as described below, and the routine use of insufflation additionally benefits robotic surgeons during segmentectomy. Herein we describe robotic surgical techniques for standard segmentectomies, including bilateral superior (S6) and basilar segmentectomies (S7-10), left upper lobe trisegmentectomy (S1-3), and lingulectomy (S4-5).
Preoperative evaluation
Appropriate patient selection for robotic-assisted segmentectomies is crucial to minimize open conversions and to ensure an oncologically sound operation. Standard preoperative assessment and staging are performed. A high-resolution chest and upper abdomen CT with contrast is particularly valuable for a precise understanding of the anatomical location of the tumor and the pulmonary arterial branches to the segment of interest. The size, location, and relationship of the tumor to intersegmental veins is studied in detail to plan the segmental anatomic resection and ensure appropriate margins. Particular attention is also given to any calcified lymph nodes that may be encountered during the dissection as this may increase the likelihood of pulmonary arterial bleeding during the operation (12). Additionally, whole body PET/CT and pulmonary function tests are obtained. According to the tumor size and PET/CT findings, appropriate patients undergo preoperative pathologic mediastinal staging with either endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) or mediastinoscopy.
Patient positioning, port placement, and docking
The patient is intubated in the supine position with planned selective lung ventilation. A double-lumen endotracheal tube is preferred, though a bronchial blocker is an acceptable alternative. The patient is then positioned in the lateral decubitus position. Appropriate placement of the endotracheal tube is confirmed, as repositioning after docking is challenging. For the da Vinci Xi Surgical System (Intuitive Surgical, Sunnyvale, CA) the patient’s head remains oriented towards the anesthesia station and the robot can be docked perpendicularly from either side of the patient. For the da Vinci Si Surgical System, the patient is turned 90 degrees and the robot is docked over the patient’s shoulder approximately 15 degrees off the patient’s longitudinal axis (13).
Our preference is to place ports along a single intercostal space, generally the 8th intercostal space similar to previously described placement (13). A theoretical advantage of this approach is minimizing damage to multiple intercostal nerves by limiting ports to the same interspace. However, this strategy is modified as necessary according to patient characteristics in order to maintain at least 8 cm spacing between ports. In patients with more pronounced rib angulation, the posterior port is often dropped to the 9th intercostal space to allow for improved working room of the posterior arm. For petite patients with a shortened thorax, the anterior port may be brought up to the 7th intercostal space. Additionally, in smaller patients with limited surface area the ports may be placed with as little as 6 cm between them, though any closer risks collisions between the robotic arms. We have moved entirely to the Xi system for thoracic operations, and our described technique is based on this platform. We use either three 8 mm ports and one anterior 12 mm port for stapling, or two 8 mm ports with one 12 mm port anteriorly and one 12 mm port posteriorly to facilitate stapling. The 8 mm camera port is placed along the posterior axillary line in the 8th intercostal space. The 0-degree camera is favored to minimize pain that can occur with 30-degree angled cameras from torque against the intercostal bundle. CO2 insufflation is utilized to assist with lung collapse and to displace the diaphragm inferiorly. A multilevel posterior intercostal nerve block is performed with liposomal bupivacaine, or alternatively, bupivacaine with epinephrine can be safely used. The most posterior 8 mm port is placed 4–5 cm lateral to the transverse process of the vertebral body. The second 8 mm port is placed halfway between the camera and posterior port. This port can be up-sized to 12 mm to allow stapling from a posterior angle if needed. The anterior 12 mm port is placed as far anteriorly as possible and serves as the stapling port. An assist port can be placed one or two rib spaces inferior and anterior to the camera, triangulated with the 12 mm port. The robot is then docked and the targeting feature can then be used to assist with optimal boom rotation, and in general the boom is rotated to ensure the arms are parallel to the line of trocars. On the Xi system, arms are docked parallel to each other, about one fist length apart, and the patient clearance joint is rotated downward. We favor the use of a small grasping retractor in the posterior arm, fenestrated bipolar forceps in the left hand, and the curved bipolar dissector in the right hand. We find the curved bipolar allows precise dissection and more controlled electrocautery near the pulmonary artery (PA). Alternatively, others routinely use hook or spatula cautery with great success (11,14).
General operative considerations
Regardless of which segmentectomy is performed, each case is begun with the same general steps. The pleural surface is inspected for evidence of metastases and, while careful not to grasp the tumor, its location is confirmed. The mediastinal lymph node dissection is performed first, and all lymph nodes are routinely sent for frozen section analysis (13). If any return positive, then a lobectomy is performed. The inferior pulmonary ligament is divided and the inferior pulmonary vein identified. Station 9 followed by visible station 8 lymph nodes are removed. The pleura along the posterior hilum is divided and station 7 lymph nodes are removed. For right-sided segmentectomies, station 4R is then removed. On the left side, station 5 and 6 are removed, cognizant to avoid injury to the vagus/recurrent laryngeal nerves. Additional detail regarding techniques for mediastinal lymph node dissection can be found elsewhere in this focused issue. We then proceed with dissection for the planned segmentectomy, beginning with the station 11 and 12 lymph nodes to the target segment. These are sent for frozen section analysis, and we convert our plan to a lobectomy if metastatic disease to any N1 lymph nodes is confirmed. Specific segmental resections are discussed in greater detail below. Table 1 provides a reference for keys to dissection and potential pitfalls for each of the standard segmentectomies.
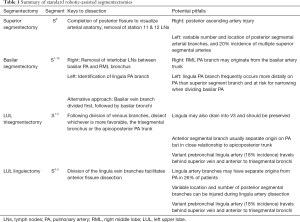
Full table
Operative technique for standard segmentectomies
Right and left lower lobe superior segmentectomy (S6)
After the N1 nodes are removed as previously described, the completeness of the fissure is assessed. If the fissure is complete or near complete, dissection is started within the oblique fissure overlying the PA. The exceptional visualization afforded by the robotic approach allows for fissure dissection with little parenchymal injury. The station 12 lymph nodes on either side of the PA are identified and removed. This facilitates identification of the superior border of the superior segmental artery. If the remaining fissure is thin posteriorly then continued bipolar dissection can be used to complete it. However, if the lung parenchyma is well developed in this location then blunt dissection can be used to develop a window to the posterior hilum.
On the right, dissection should proceed cautiously to avoid injury to the posterior ascending artery which lies just above, and is placed under tension due to cephalad retraction of the right upper lobe. Alternatively, a posterior to anterior dissection may be performed to create a plane for completion of the posterior fissure (13). To do so, identify the takeoff of the right upper lobe bronchus at its inferior aspect along the bronchus intermedius. Careful dissection at this location will lead to the station 11 lymph node (“sump node”) which obscures the posterior ascending artery. Removal of the node facilitates identification of not only the posterior ascending but also the more inferiorly located superior segmental artery. Dissection between these two vessels can be performed to develop a plane beneath the parenchyma in the posterior fissure. The fissure can then be partially divided to expose the underlying vessels. Of note, if an incomplete fissure is identified at the outset of the case, this is our preferred initial dissection to gain access to the interlobar PA.
For a left superior segmentectomy, care must be taken when dissecting around the PA, as 20% of patients have multiple lingular branches, and total PA branches can vary from 2 to 7 to the left upper lobe (15,16). Similar to the right side, if the posterior fissure is thin, it may be completed with bipolar electrocautery. Alternatively, a window to the posterior hilum can be created overlying the PA through either an anterior or posterior approach. Clear visualization of the dissection plane is necessary to identify and avoid any left upper lobe PA branches that may be under tension from retraction of the left upper lobe.
With the posterior fissure completed, the superior segmental artery is now clearly seen, and can be further dissected and divided. Both the right and left superior segmental arteries most frequently originate as a single vessel. However, either side can have multiple branches. This more commonly occurs on the left, with up to 20% of patients demonstrating two or even three branches (16). The superior segmental bronchus is then readily identified. Prior to dividing the airway, the superior segmental vein is divided to facilitate bronchial dissection. To do so, the lung is flipped anteriorly, and dissection along the superior edge of the inferior pulmonary vein reveals the superior segmental vein branching cephalad. After dividing the vein, the superior segmental bronchus can be further cleared of any nodal tissue and skeletonized. If any uncertainty exists regarding the airway anatomy, a compression-inflation test can be performed for verification. The superior segmental bronchus is stapled at its takeoff. The intersegmental plane is identified and the parenchyma is divided with staplers (see below for further discussion of techniques to delineate the intersegmental plane).
Right and left lower lobe basilar segmentectomy (S7-10)
Multiple approaches may be utilized for basilar segmentectomy. If the fissure is well-developed then dissection may begin similar to superior segmentectomy, within the oblique fissure overlying the PA. On the right, it is particularly important to remove the interlobar lymph nodes separating the anteromedial side of the basilar PA from the right middle lobe bronchus. On the left, it is important to identify the lingular PA branch to avoid narrowing as its origin occurs anteriorly and more distally on the PA than the superior segmental artery. Dissection is carried anteriorly along the PA and the anterior fissure is dissected free and completed. If difficulty is encountered with this dissection, then the lung can be flipped posteriorly and an anterior to posterior approach can be used to help complete the anterior fissure. With the basilar PA branches well exposed, a stapler may be passed from the anterior port and used to divide these branches, taking care to preserve the superior segmental artery. Occasionally on the right, the right middle lobe PA branch originates low on the basilar artery trunk and care must be taken to ensure the middle lobe artery is preserved (16). To facilitate visualization of the basilar airways, the lung is retracted anteriorly, the inferior pulmonary vein is dissected, and the branch draining the superior segment is identified cephalad. Preserving this branch, the common vein to the basilar segments is stapled. The basilar segmental bronchi are then identified and dissected. A compression-inflation test can be performed for verification. The basilar segmental bronchi are stapled, the intersegmental plane is identified, and the parenchyma is divided with staplers.
An alternative and often more straightforward approach, regardless of the type of fissure, is to divide the basilar vein branches first. Doing so affords exposure to the basilar bronchi. After dividing the basilar bronchi, the segmental arterial branches to the lower lobe are easily identifiable. Division of the PA branches to the basilar segments is followed by stapled division of the anterior fissures and parenchyma along the intersegmental plane. The drawback to this approach is that the interlobar nodal dissection, and subsequent frozen section analysis, occurs later in the operation, leading to potential delays while waiting for pathologic results.
Left upper lobe trisegmentectomy (S1-3)
For these cases, we typically utilize a 12 mm port in the 2nd from most posterior arm for ease in stapling the upper division veins and first apical PA branch. After completing the N1 lymphadenectomy, dissection is continued posteriorly within the oblique fissure overlying the PA, careful to avoid injury to the left upper lobe posterior segmental PA branches. The posterior fissure is completed with electrocautery or stapled. The pleura over the apex of the hilum is mobilized if not previously done during the station 5 nodal dissection. Station 12L lymph nodes are removed, and the posterior segmental PA branches are divided. The lung is retracted posteriorly, the pleura is divided over the superior pulmonary vein, and any station 10L lymph nodes are removed. The superior edge of the superior pulmonary vein is defined and carefully dissected away from the apicoposterior PA trunk. The overlying pleura and lung parenchyma is gently dissected off the superior pulmonary vein to visualize the branching pattern. There are generally three main venous branches, V1-2 and V3 to the apicoposterior and anterior segments, respectively, and V4-5 to the lingula. However, in some instances V4-5 is small and part of the lingula drains into V3 (16). In these cases, we preserve V3 with V4-5 in order to minimize the risk for congestion of the lingua. The trisegmental venous branches are encircled and stapled. Either the trisegmental bronchus or the apicoposterior PA trunk is dissected next, depending on which anatomy is more favorable. In either case, care must be taken not to injury the anterior segmental PA branch, as it more frequently arises separately off the PA and may be in close proximity. Another important variant is the mediastinal or prebronchial lingular artery (18% incidence), which arises proximally near the apicoposterior trunk then travels along the anterior hilum between the superior pulmonary vein and trisegmental bronchi (16). For division of the trisegmental bronchial trunk, the bronchus must be dissected until the lingular bronchus can clearly be identified. Any lymph nodes are removed and sent for frozen section analysis. Compression-inflation may be performed to confirm bronchial anatomy, and the trisegmental trunk is stapled. The anterior segmental PA branch will now clearly be visible if it was not previously, and is divided. The intersegmental plane is identified and the parenchyma divided with staplers.
Left upper lobe lingulectomy (S4-5)
The dissection begins in the fissure overlying the PA. Station 11L and 12L nodes are dissected free and sent for frozen section analysis. The fissure is dissected from posterior to anterior, and may be completed with bipolar electrocautery or stapled. If incomplete, then an anterior to posterior approach can be performed by first dissecting the superior pulmonary vein, isolating, and then dividing the lingula venous branch. Occasionally, the lingula drains into the inferior pulmonary vein and is similarly divided. Division of the lingular vein branches exposes the left upper lobe bronchus. Safe completion of the anterior fissure is facilitated by removal of a characteristic level 11 lymph node at the secondary carina. Removal of this node allows the surgeon to obtain access to the subadventitial plane of the PA and to complete the anterior fissure with a stapler. The lingula PA branch arises anteriorly off the interlobar PA and is exposed by elevating the lingula anteriorly and cephalad. It generally arises as a single trunk, but 26% of patients will have separate segmental origins from the PA (16). Additionally, care must be taken when dissecting around the lingula PA branch, as posterior segmental arterial branches may be located nearby. The station 12L lymph nodes located between the trisegmental bronchus and the lingula bronchus are removed. The lingula bronchus is isolated and divided. The intersegmental plane is then identified and divided with staplers.
Strategies for intersegmental plane identification
There are several strategies for identifying the parenchymal resection plane for an anatomic segmentectomy. The most conventional method utilizes deflation of the target segment. After the bronchus is clamped or divided, the remaining lung parenchyma is inflated, and the intersegmental plane is identified. While easy to perform, this method limits the surgeon’s view during robotic surgery significantly. Additionally, the target segment may inflate by collateral ventilation and trapped air, thereby obscuring the intersegmental plane. Furthermore, subsequent deflation of the lung prior to division of the parenchyma is often time consuming, particularly in emphysematous patients (17). These drawbacks may contribute to poor identification of the true intersegmental plane, and subsequently a closer parenchymal margin to the tumor (18). Our preferred method leverages the da Vinci’s Firefly near-infrared imaging capability and the fluorescence of indocyanine green (ICG). Following division of the segmental PA branches, 12.5 mg ICG (25 mg diluted with 10 mL sterile water, 5 mL per injection followed immediately by 10 mL saline flush) is injected intravenously by the anesthesia team, resulting in the perfused lung fluorescing green under near-infrared light (19). The segment of interest lacks fluorescence given the divided blood supply. The intersegmental plane is visualized with Firefly and marked with electrocautery. While the fluorescence only lasts a brief period, there is generally enough time to mark the plane, and the ICG injection can be repeated if necessary. An additional benefit of using ICG is that unexpectedly devascularized lung tissue can be identified and included in the resection, thereby avoiding potentially infarcted lung parenchyma being left behind (17). Alternatively, ICG may also be injected transbronchially directly into the target segment with similar results (20). Additional novel strategies using CT imaging and bronchoscopy have recently been developed. Virtual assisted lung mapping and electromagnetic navigational bronchoscopy use three-dimensional virtual images to guide bronchoscopic marking of the lung to facilitate the identification of tumors and intersegmental resection planes (21,22).
Discussion
There are distinct advantages of using the da Vinci robotic platform to perform anatomic pulmonary segmentectomies. The three-dimensional visualization with a stable camera platform allows for fine dissection of lymph nodes around the PA, and the wristed movements and maneuverability permits dissection from multiple different approaches. These benefits open up further possibilities for complex segmentectomies that are discussed in depth elsewhere in this special issue. The first series of robotic segmentectomies was published in 2011, and since then, only a handful of retrospective studies and no randomized studies have been published. The largest series of 100 patients published in 2017 by Drs. Wei and Cerfolio demonstrated excellent results, with only 7% converted to lobectomy, mean operative time of 88 minutes, a 3-day median length of stay, 2% rate of postoperative complications, and no mortality events at 60 days (13). A meta-analysis by Liang et al. published in 2018 compared robotic-assisted lobectomies and segmentectomies (RAL/S) to video-assisted thoracoscopic surgery (VAL/S). This study demonstrated a lower 30-day mortality rate for RAL/S (0.7%) compared to VAL/S (1.1%; P=0.045) and lower conversion rate to open surgery of RAL/S (10.3%) compared to VAL/S (11.9%; P<0.001). The postoperative complication rate, operative duration, hospital length of stay, days to chest tube removal, number of lymph nodes and nodal stations retrieved were all similar between the two groups (23). These studies suggest robotic-assisted pulmonary resections can be performed safely, and with equivalent or better outcomes, compared to VATS. As adoption grows and surgeons become increasingly proficient in robotic techniques, operative times and costs may also improve.
The ongoing debate regarding whether anatomic segmentectomies are an oncologically sound operation will likely have a major impact on the utilization of robotic-assisted segmentectomies. Two phase III randomized clinical trials that attempt to address whether sublobar resections are non-inferior to lobectomy for peripheral, early stage NSCLC patients are currently ongoing. The National Cancer Institute Cancer and Leukemia Group B 140503 study (CALGB/Alliance 140503) has completed enrollment. A total of 701 patients with single, peripheral (outer third of lung), ≤2 cm NSCLC tumors were randomized to either lobectomy or sublobar resection (wedge or segmentectomy) intra-operatively after frozen section confirmed N0 status (24). Initial perioperative morbidity and mortality results were published in 2018, and demonstrated no significant difference between lobectomy and sublobar resections with regard to mortality at 30 and 90 days (1.1% and 0.6% at 30 days, 1.7% and 1.2% at 90 days, respectively), overall adverse events (54% and 51%, respectively), severe adverse events (grade ≥3, 15% and 14%, respectively), or cardiac or pulmonary complications (25). Outcomes regarding disease-free survival, overall survival, loco-regional and systemic recurrence, and pulmonary function at 6 months are expected to be reported in 2021 (24). The Japan Clinical Oncology Group (JCOG) and the West Japan Oncology Group (WJOG) study JCOG0802/WJOG4607L has also completed enrollment of 1,106 patients with peripheral NSCLC tumors ≤2 cm, who were randomized to either lobectomy or segmentectomy (26). Although the overall survival and secondary endpoint results are not yet published, Suzuki et al. recently published perioperative outcomes. Neither the lobectomy nor segmentectomy arm had any perioperative mortality events, and there were no differences in overall complications reported (grade ≥2, 26.2% in lobectomy and 27.4% in segmentectomy). However, prolonged air leaks >7 days occurred more frequently in segmentectomy patients (6.5%) compared to those undergoing lobectomy (3.8%) (2).
The results from each of these trials will help further define the role of segmentectomy, irrespective of operative approach. Understanding robotic-assisted surgical techniques to perform segmentectomies in a safe and oncologically sound manner is essential to achieving outcomes comparable to VATS and open approaches.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Alper Toker) for the series “Robotic Segmentectomies” published in Video-Assisted Thoracic Surgery. This article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2020.03.01). The series “Robotic Segmentectomies” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nakazawa S, Shimizu K, Mogi A, et al. VATS segmentectomy: past, present, and future. General Thoracic and Cardiovascular Surgery 2018;66:81-90. [Crossref] [PubMed]
- Suzuki K, Saji H, Aokage K, et al. Comparison of pulmonary segmentectomy and lobectomy: Safety results of a randomized trial. J Thorac Cardiovasc Surg 2019;158:895-907. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Lederle FA. Lobectomy versus limited resection in T1 N0 lung cancer. Ann Thorac Surg 1996;62:1249-50. [Crossref] [PubMed]
- Cao C, Chandrakumar D, Gupta S, et al. Could less be more?-A systematic review and meta-analysis of sublobar resections versus lobectomy for non-small cell lung cancer according to patient selection. Lung Cancer 2015;89:121-32. [Crossref] [PubMed]
- Guo J, Liu Y, Tian X, et al. Less is more in solid-dominant lung cancer? Sublobar resection versus lobectomy for solid-dominant stage IA non-small-cell lung cancer: A meta-analysis study. Mol Clin Oncol 2019;11:465-73. [Crossref] [PubMed]
- Wei B, D’Amico TA. Thoracoscopic Versus Robotic Approaches: Advantages and Disadvantages. Thoracic Surgery Clinics 2014;24:177-88. [Crossref] [PubMed]
- Kumar A, Asaf BB. Robotic thoracic surgery: The state of the art. J Minim Access Surg 2015;11:60-7. [Crossref] [PubMed]
- Papoulidis P, Nardini M, Dunning J. Is robot assisted thoracic surgery better than video assisted? Video-assist Thorac Surg 2017;2:74. [Crossref]
- Solinas M, Novellis P, Veronesi G. Robotic is better than VATS? Ten good reasons to prefer robotic versus manual VATS surgery in lung cancer patients. Video-assist Thorac Surg 2017;2:60. [Crossref]
- Pardolesi A, Park B, Petrella F, et al. Robotic anatomic segmentectomy of the lung: technical aspects and initial results. Ann Thorac Surg 2012;94:929-34. [Crossref] [PubMed]
- Cao C, Cerfolio RJ, Louie BE, et al. Incidence, Management, and Outcomes of Intraoperative Catastrophes During Robotic Pulmonary Resection. Ann Thorac Surg 2019;108:1498-504. [Crossref] [PubMed]
- Cerfolio RJ, Watson C, Minnich DJ, et al. One Hundred Planned Robotic Segmentectomies: Early Results, Technical Details, and Preferred Port Placement. Ann Thorac Surg 2016;101:1089-95; Discussion 1095-6. [Crossref] [PubMed]
- Choe G, Park B. Robotic-assisted thoracoscopic surgery (RATS) lobectomy. Ann Cardiothorac Surg 2019;8:296-9. [Crossref] [PubMed]
- Kandathil A, Chamarthy M. Pulmonary vascular anatomy & anatomical variants. Cardiovasc Diagn Ther 2018;8:201-7. [Crossref] [PubMed]
- Gossot D, Seguin-Givelet A. Anatomical variations and pitfalls to know during thoracoscopic segmentectomies. J Thorac Dis 2018;10:S1134-44. [PubMed]
- Sato M, Murayama T, Nakajima J. Concepts and techniques: how to determine and identify the appropriate target segment in anatomical pulmonary segmentectomy? J Thorac Dis 2019;11:972-86. [Crossref] [PubMed]
- Mehta M, Patel YS, Yasufuku K, et al. Near-infrared mapping with indocyanine green is associated with an increase in oncological margin length in minimally invasive segmentectomy. J Thorac Cardiovasc Surg 2019;157:2029-35. [Crossref] [PubMed]
- Pardolesi A, Veronesi G, Solli P, et al. Use of indocyanine green to facilitate intersegmental plane identification during robotic anatomic segmentectomy. J Thorac Cardiovasc Surg 2014;148:737-8. [Crossref] [PubMed]
- Sekine Y, Ko E, Oishi H, et al. A simple and effective technique for identification of intersegmental planes by infrared thoracoscopy after transbronchial injection of indocyanine green. J Thorac Cardiovasc Surg 2012;143:1330-5. [Crossref] [PubMed]
- Bolton WD, Howe H 3rd, Stephenson JE. The utility of electromagnetic navigational bronchoscopy as a localization tool for robotic resection of small pulmonary nodules. Ann Thorac Surg 2014;98:471-5; discussion 475-6. [Crossref] [PubMed]
- Sato M, Omasa M, Chen F, et al. Use of virtual assisted lung mapping (VAL-MAP), a bronchoscopic multispot dye-marking technique using virtual images, for precise navigation of thoracoscopic sublobar lung resection. J Thorac Cardiovasc Surg 2014;147:1813-9. [Crossref] [PubMed]
- Liang H, Liang W, Zhao L, et al. Robotic Versus Video-assisted Lobectomy/Segmentectomy for Lung Cancer: A Meta-analysis. Ann Surg 2018;268:254-9. [Crossref] [PubMed]
-
Comparison of Different Types of Surgery in Treating Patients With Stage IA Non-Small Cell Lung Cancer - Altorki NK, Wang X, Wigle D, et al. Perioperative mortality and morbidity after sublobar versus lobar resection for early-stage non-small-cell lung cancer: post-hoc analysis of an international, randomised, phase 3 trial (CALGB/Alliance 140503). Lancet Respir Med 2018;6:915-24. [Crossref] [PubMed]
- Nakamura K, Saji H, Nakajima R, et al. A phase III randomized trial of lobectomy versus limited resection for small-sized peripheral non-small cell lung cancer (JCOG0802/WJOG4607L). Jpn J Clin Oncol 2010;40:271-4. [Crossref] [PubMed]
Cite this article as: White PT, Antonoff MB, Rajaram R. Robotic surgical techniques for standard segmentectomies. Video-assist Thorac Surg 2020;5:37.


