
Survival after video-assisted thoracoscopic surgery for lung metastasectomy
Introduction
Lung metastases develop in about 30% of patients with malignant solid tumors (1). In selected patients with disease confined to the lungs and good pulmonary reserve, surgical resection represents the treatment of choice, with 5-year survival rates ranging from 20% to 50% (1). There is a general agreement regarding the following points: (I) wedge resection is the most common procedure; (II) lobectomy and pneumonectomy is recommended for larger or centrally located lesions; and (III) lymph node sampling/dissection is more useful for diagnostic purposes and predicting patient prognosis than as curative technique (2). However, the best surgical approach is still disputed.
Since its introduction in the early 1990s, video-assisted thoracoscopic surgery (VATS) has become the preferred approach among thoracic surgeons for management of early stage lung cancer. However, VATS seem to be not sufficiently robust to find as many nodules as open thoracotomy and, consequently, its oncological validity remains inferior if the intention is to clear all pulmonary metastases during surgery (3,4). Thus, thoracotomy approach still remains the preferred strategy for pulmonary metastasectomy. Despite all, in the last decade the use of VATS continues to grow among thoracic surgeons for performing curative pulmonary metastasectomy because of smaller and less painful incision, shorter hospital stay, and fewer morbidity and mortality compared to thoracotomy (5).
Thus, this review aimed to evaluate the outcome of patients undergoing resection of lung metastasis performed using VATS in order to establish whether this approach did not compromise oncological results. We present the following with the PRISMA reporting checklist (available at http://dx.doi.org/10.21037/vats-2020-lm-02).
Methods
Search strategy
The study design was structured according to the PRISMA protocol (6). A literature review was carried out using MEDLINE, PubMed, Scopus, Google Scholar, and Cochrane databases until the end of March 2020 in order to label all studies that evaluated VATS resection as treatment for pulmonary metastases. The following MeSH search headings were used: (‘pulmonary’ or ‘lung’) and (‘metastasectomy’ or ‘metastatectomy’ or ‘metastectomy’ or ‘metastectomy’ or ‘metastasis’ or ‘metastases’) and (‘VATS’ or ‘thoracoscopy’ or ‘thoracoscopic’ or ‘thoracotomy’ or ‘thoracic surgery’). Additional papers, abstracts, chapter of books, abstracts, letters and editorials were retrieved from bibliographies by manual research. The Science Citation Index was used to cross reference for further studies that met our criteria. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). None of registration number of ethics board and individual consent for this retrospective analysis were needed for this paper.
Selection process
Papers were included in the review if they fit the following criteria: (I) papers published in English; (II) study population including patients undergoing lung metastasectomy performed via VATS; (III) results that reported survival and/or recurrence. We excluded (I) studies not published in English; (II) reviews, metanalyses, abstracts, case reports and case series; (III) papers from the same groups or based on the same population. In these cases, only the most recent publication was reported to avoid duplication; and (IV) papers reported pulmonary metastasectomy through robotic approach.
Five reviewers analyzed all selected articles. First, the titles of papers were inspected to decide whether they were appropriate to the research purpose. Then, the abstracts of the selected papers were evaluated, and those that were not appropriate were excluded. Finally, the remaining articles were entirely inspected to decide their inclusion in the analysis based on the above reported criteria. Disagreements were judged by the two senior reviewers (AF and MS) after referring to the original articles.
Results
The flow chart of the study was reported in Figure 1. A total of 598 articles were selected using the above reported databases (n=579), and the additional manual (n=20) searches from the references of the selected articles. Four hundred fifty-seven papers were excluded as duplicate. Thus, 142 papers were screened, and 110 studies were excluded after examination of the title and abstracts. The remaining 32 studies were cautiously evaluated by all authors, who excluded further 22 studies. Thus, our review included a total of 10 papers. The following data were extracted from the selected papers: the authors, the year of publication, the country, the study design, the study population, the outcomes, and the study limitations.
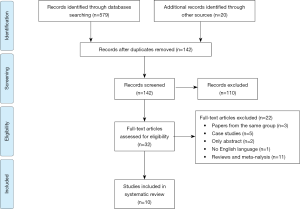
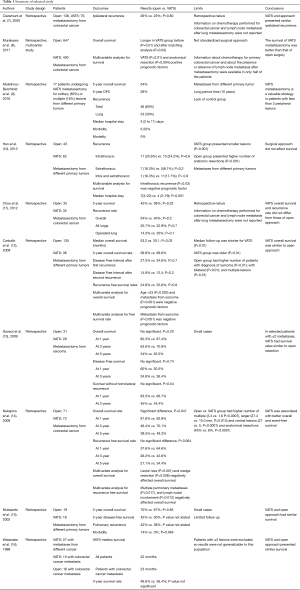
Our review included 10 retrospective studies listed in Table 1 (7-16). Five articles evaluated pulmonary metastases from colorectal cancer only, one from sarcoma only; and four from different primary cancers. In 9 out of 10 studies VATS outcomes were compared with those of thoracotomy.
Full table
Claramunt et al. (7) retrospectively evaluated the data of 211 patients with colorectal cancer undergoing pulmonary metastasectomy performed by open (n=136), and VATS approach (n=75). The two groups were not balanced as open group presented higher rates of patients with cardiovascular risk factor (P=0.003), and this strategy was used for resecting multiple nodules (P<0.0001) while VATS for a single peripheral nodule (P=0.0003). To balance these differences, a propensity score matching analysis was performed considering the following covariates as age, sex, presence of a cardiovascular risk factor, presence of a respiratory risk factor, Disease Free Survival, number of metastases and type of resection. The authors found no significant difference in ipsilateral recurrence rates between open and VATS surgery (26% vs. 23%; P=0.80). Information on chemotherapy performed for colorectal cancer, and on lymph node metastasis after lung metastasectomy were not reported.
Murakawa et al. (8) in a multi-institutional, retrospective study evaluated the data of 1,047 patients who underwent lung metastasectomy for colorectal cancer using open (n=647) or VATS approach (n=400). Overall survival was compared between two study groups before and after matching analysis. Then, multivariable analysis was used to determinate prognostic survival factors. The VATS group presented a better survival than open group (P=0.01), and these results were confirmed also after adjusting for the propensity score (P=0.03). VATS (P=0.01), and anatomical resection (P=0.004) were the only positive prognostic factors for survival. The indications for surgery and for the type of resection were not standardized, but they were based on decisions of each participated centers. Yet, information on chemotherapy performed for the colorectal cancer, and on lymph nodes involvement after lung metastasectomy were available only in half of patients.
Abdelnour-Berchtold et al. (9) retrospectively reviewed the data of 77 patients operated of pulmonary metastasectomy using VATS for solitary (n=63) or multiple (n=14) lung metastases from different primary cancers as colorectal carcinoma (n=26), sarcoma (n=17), melanoma (n=16), or others tumors (n=18). Among these, 9 patients had bilateral lesions treated with synchronous (n=4) or sequential (n=5) resections. In all cases, the lesions were identified by thin-section chest computed tomography scans. The median hospital stay was 3 (2 to 11) days; the postoperative mortality was 0% and postoperative morbidity was 5.2%. Recurrence was found in 46 (60%) patients. Of these, 23 (30%) had recurrence within lung, and 8 patients (10%) in the operated lungs. Seven out of eight patients underwent re-metastasectomy by VATS (n=5) or thoracotomy (n=2). The overall 5-year survival rate was 54%. Patients with recurrence treated by re-metastasectomy and those without recurrence had similar survival. The different primary tumors, the long period time (about 10 years), and the lack of a control group (i.e. , open metastasectomy) are the main limits of this paper.
Hann et al. (10) retrospectively compared the data of 105 patients underwent solitary lung metastasectomy due to different primary tumors. Patients were divided in two groups based whether metastasectomy was performed using open (n=43) or VATS approach (n=62). In all cases, the lesions were identified by thin-section chest computed tomography scans. Compared to the open group, the VATS group had a shorter median postoperative hospital stay (7 vs. 4; P<0.001), while no significant differences were found regarding intrathoracic (25.6% vs. 24.2%; P=0.6); extrathoracic (16.3% vs. 8.1%; P=0.2); and intrathoracic plus extrathoracic (16.3% vs. 17.7%; P=0.8) recurrences. Eight patients (18.6%) in the open group and 11 patients (17.7%) in the VATS group (P=0.9) underwent re-metastasectomy. Overall survival did not differ between the two groups (P=0.210). Intrathoracic recurrence was the only significant risk factor for overall survival on multivariable analysis (P=0.03). There is selection bias that could affect the results. VATS group presented smaller lesions than open group (12 vs. 25 mm; P<0.001), while open group presented higher numbers of anatomic resection (19 vs. 5; P<0.001).
Chao et al. (11) evaluated the data of 143 patients with colorectal cancer undergoing pulmonary metastasectomy performed by open (n=53), and VATS approach (n=90). After being matched for tumor number, size, and type of resection, 35 of patients for each group were analyzed. No significant differences were found between open and VATS group related to overall recurrences (54 vs. 40%; P=0.2), all lung recurrences (25.7 vs. 22.9%, P=0.7), same side lung recurrences (14.3 vs. 20%, P=0.7), and 5-year overall survival (43% vs. 51%; P=0.21). Information on chemotherapy performed for colorectal cancer and on lymph node metastasis after lung metastasectomy were not reported.
Carballo et al. (12) evaluated the data of 171 patients undergoing lung metastasectomy performed by open (n=135) or by VATS (n=36) approach. Primary cancers included sarcoma (n=81; 47%), colorectal adenocarcinoma (n=26; 15%) and renal cell carcinoma (n=22; 13%). Thoracotomy and VATS group presented a 5-year overall survival rates of 58.8% and of 69.6%, respectively (P=0.27), and a median overall survival of 53.2 and 30.1 months, respectively (P=0.03). Second recurrences occurred in 59 thoracotomy patients, and in 10 VATS patients. Multivariable analysis showed that age >53 years old (P=0.002) and metastases from sarcoma (P<0.001) were negative prognostic factors for overall survival. There is selection bias that could affect the results. Median follow-up was significant shorter for VATS group (P=0.01). VATS patients were older (P<0.01) while open group presented higher number of patients with metastasis from sarcoma (P<0.01); and with bilateral (P<0.01); and multiple lesions (P<0.01).
Gossot et al. (13) retrospectively evaluated the data of 60 patients with lung metastases from sarcoma undergoing metastasectomy performed by open (n=29) or VATS approach (n=31). Open compared to VATS group was associated with longer hospital stay (6.2 vs. 3.7 days, respectively, P<0.0001). VATS compared to open group showed no significant difference (P=0.20) regarding overall survival rates at 1 (87.4% vs. 82.3%, respectively), 3 (70.9% vs. 63.6%, respectively), and 5 (52.5% vs. 34%, respectively) years; and regarding disease-free survival rates (P=0.74) at 1 (50.5% vs. 60%, respectively) and 3 (26.4% vs. 24.8%, respectively) years. Only one patient in each group had local recurrence. VATS compared to open group showed no significant difference (P=0.54) regarding survival without a homolateral recurrence at 1 (66.7% vs. 83.5%, respectively), and 3 (44.4% vs. 45%, respectively) years.
Nakaijima et al. (14) evaluated the data of 143 patients with lung metastases from colorectal cancer. Patients were splitted into two groups, based on whether the resection of metastases was performed by VATS (n=72) or open (n=71) approach. VATS compared to open group was associated with higher 5-year overall survival rate (49.3% vs. 39.5%, respectively, P=0.047) and 5-year recurrence free survival rate (34.4% vs. 21.1%, respectively, P=0.064). We found no significant difference in the survival rates between open and VATS groups, even with elimination of the patients with multiple pulmonary metastases in both groups. Multivariable analysis found that lesion size (P<0.001); and wedge resection (P=0.026) negatively influenced overall survival, while multiple pulmonary metastases (P=0.017), and lymph nodal involvement (P=0.012) negatively influenced recurrence-free survival. VATS did not influence overall (P=0.29) and recurrence-free survival (P=0.18). There is selection bias that could affect the results. Open compared to VATS group had higher number of multiple (3.4 vs. 1.6, respectively, P=0.0007), larger (27.4 vs. 15.0 mm, respectively, P=0.015) and central lesions (27 vs. 5, respectively, P<0.0001), and of anatomical resections (45% vs. 8%, respectively, P<0.0001).
Mutsaerts et al. (15) evaluated the data of 35 patients who underwent VATS metastasectomy with (n=19) or without (n=16) confirmatory thoracotomy. VATS compared to open group was associated with lesser postoperative morbidity (0 vs. 14%, respectively, P=0.049). Open compared to VATS approach showed no significant difference regarding 2-year overall survival (70% vs. 67%, respectively, P=0.85); 2-year disease-free survival (42% vs. 50%, respectively); pulmonary recurrence (42% vs. 38%, respectively). Only 20 patients were available for survival analysis, and that limited the results.
Watanabe et al. (16) retrospectively compared patients undergoing VATS resection of colorectal metastases (n=15) with a historical cohort of patients undergoing open resections (n=16). VATS compared to open group showed no significant difference in 3-year survival rates (56.4% vs. 48.6%, respectively). Patients with ≥3 metastases were excluded, and that could affect the results.
Discussion
The main objective of pulmonary metastasectomy is to obtain the complete resection of disease while preserving pulmonary parenchyma as much as possible. The International Registry of Lung Metastases (17) has reported that patients with complete resection had higher 5-year survival rates that those with incomplete resection (36% vs. 13%, respectively). Despite finger inserted through the port holes could palpate lung parenchyma during VATS, this strategy still presents limit in detecting small and deep lesions. Retrospective and prospective studies (3,4) have reported that bimanual lung palpation during thoracotomy could discover small nodules missed by the chest CT. Thus, open approach is still considered the treatment of choice to obtain complete resection of all pulmonary metastases during surgery. However, in the last decade there has been a re-examination of the use of VATS for performing pulmonary metastasectomy. A survey among members of the ESTS demonstrated that 40% now used VATS for therapeutic pulmonary metastasectomy (5). There are different arguments that could limit the benefits of thoracotomy over VATS in this setting. (I) New radiological and surgical technologies allow to detect and resect small and deep pulmonary nodules missed by conventional CT (18). (II) The excision of all digitally detected micronodules did not provide a demonstrated survival benefits as one third of these lesions are benign. Yet, recurrent pulmonary metastatization may be resected with satisfying results in selected cases. (III) An open thoracotomy may allow bimanual palpation of only one lung, but the most of postoperative recurrence occurred within contralateral lung or in extra-pulmonary sites. (IV) During VATS it is possible to detect pleural implants, that would stop the surgeon to proceed with the resection before performing thoracotomy.
The results of our review supported this tendency showing that in selected cases the oncological results of VATS are not inferior to those of thoracotomy. In fact, 9 (7,9-16) out of the ten reviewed studies compared survival and/or recurrence rates between VATS and thoracotomy approach. No significant differences were found in 7 studies (7,10,11,12,13,15,16), while in two (8,14) VATS was associated with a better overall survival. The remaining one study (9) evaluated VATS survival alone. The authors reported an overall 5-year survival rate of 54%, that was comparable to the survival expected for metastasectomy by thoracotomy. Multivariable analysis for detecting prognostic factors for overall survival or recurrence were performed in four studies (8,10,12,14). In one study (8), VATS was correlated to better survival while in the other three (10,12,14) the different surgical approach (VATS or thoracotomy) did not influence outcomes. Two studies evaluated hospital stay (10), perioperative morbidity and mortality (15) between open and VATS approach. VATS was associated with shorter hospital stay (10), and lesser perioperative morbidity (15). Our results were in line with previous reviews and metanalysis (19-25). Cheang et al. (19) found no statistically significant difference in terms of survival and recurrences between open and VATS approach, except for the 3-year overall survival in favor of VATS. Dong et al. (20) showed that VATS pulmonary metastasectomy had 1, 3, and 5-year survival rates comparable to those of thoracotomy. Patients with metastatic lung cancer were likely to relapse in the lung, and after lung metastasectomy by VATS, they might benefit from a second metastasectomy. Greenwoold et al. (21) reported no survival difference between VATS and thoracotomy, but VATS was associated with shorter hospital stay, and fewer morbidity. Meng et al. (22) found a longer survival in the VATS than in open group although not significant, and no difference was found in recurrence-free survival. Sauvain et al. (23) showed similar survival and local recurrence between VATS and open metastasectomy. Despite VATS could miss potential resectable nodules due to lack of bimanual palpation of the lungs, however in selected cases it should be considered as the strategy of choice due to the lower morbidity and length of hospital stay compared to thoracotomy. On the other hand, thoracotomy should still be suggested for patients with deep and multiple lung nodules in order to obtain complete resection. This concept was also supported by the data of Migliore et al. (24,25). Since open metastasectomy did not confer a proven better survival, VATS should be considered the preferred approach due to the advantages related to the minimal invasive nature of the procedure.
Our results should be considered with cautious before drawing definitive conclusions regarding the oncological validity of VATS. All evaluated studies are retrospective, and there are no randomized controlled trials. The differences in patient characteristics (i.e. , number of lesions, laterality, size, primary metastatic cancer type), and in extend of resection (i.e. , sublobar and lobar resections) make the VATS and open group not comparable. Furthermore, in only three studies propensity score matching analysis was performed to overcome these differences.
Conclusions
The actual literature looks to support VATS approach for metastasectomy in selected cases as one or two peripheral nodules diagnosed by thin-section chest computed tomography scan, and localized in separate lobes. However, the decision of the surgical approach should be discussed in a multidisciplinary board, and patient needs always to be aware not only of the advantages of VATS, but also of the risk of leaving undetected malignant nodules behind if VATS is performed. Only future randomized controlled trials could define the oncological validity of VATS for curative treatment of lung metastases.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Marcello Migliore and Michel Gonzalez) for the series “VATS in Lung Metastasectomy” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at http://dx.doi.org/10.21037/vats-2020-lm-02
Data Sharing Statement: Available at http://dx.doi.org/10.21037/vats-2020-lm-02
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats-2020-lm-02). The series “VATS in Lung Metastasectomy” was commissioned by the editorial office without any funding or sponsorship. AF serves as an unpaid editorial board member of Video-Assisted Thoracic Surgery from Apr 2019 to Mar 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). None of registration number of ethics board and individual consent for this retrospective analysis were needed for this paper.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Treasure T, Milošević M, Fiorentino F, et al. Pulmonary metastasectomy: what is the practice and where is the evidence for effectiveness? Thorax 2014;69:946-9. [Crossref] [PubMed]
- Higashiyama M, Tokunaga T, Nakagiri T, et al. Pulmonary metastasectomy: outcomes and issues according to the type of surgical resection. Gen Thorac Cardiovasc Surg 2015;63:320-30. [Crossref] [PubMed]
- Eckardt J, Licht PB. Thoracoscopic versus open pulmonary metastasectomy: a prospective, sequentially controlled study. Chest 2012;142:1598-602. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, McCarty TP, et al. A prospective study to determine the incidence of non-imaged malignant pulmonary nodules in patients who undergo metastasectomy by thoracotomy with lung palpation. Ann Thorac Surg 2011;91:1696-700. [Crossref] [PubMed]
- Internullo E, Cassivi SD, Van Raemdonck D, et al. ESTS Pulmonary Metastasectomy Working Group. Pulmonary metastasectomy: a survey of current practice amongst members of the European Society of Thoracic Surgeons. J Thorac Oncol 2008;3:1257-66. [Crossref] [PubMed]
- Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. [Crossref] [PubMed]
- Claramunt NP, Hwang D, de Perrot M, et al. Incidence of Ipsilateral side Recurrence after Open or VATS resection of colorectal lung metastases. Ann Thorac Surg 2020;109:1591-7. [Crossref] [PubMed]
- Murakawa T, Sato H, Okumura S, et al. Metastatic Lung Tumor Study Group of Japan. Thoracoscopic surgery versus open surgery for lung metastases of colorectal cancer: a multi-institutional retrospective analysis using propensity score adjustment. Eur J Cardiothorac Surg 2017;51:1157-63. [Crossref] [PubMed]
- Abdelnour-Berchtold E, Perentes JY, Ris HB, et al. Survival and Local Recurrence After Video-Assisted Thoracoscopic Lung Metastasectomy. World J Surg 2016;40:373-9. [Crossref] [PubMed]
- Han KN, Kang CH, Park IK, et al. Thoracoscopic resection of solitary lung metastases evaluated by using thin-section chest computed tomography: is thoracoscopic surgery still a valid option? Gen. Thorac Cardiovasc Surg 2013;61:565-70. [Crossref] [PubMed]
- Chao YK, Chang HC, Wu YC, et al. Management of lung metastases from colorectal cancer: video-assisted thoracoscopic surgery versus thoracotomy--a case-matched study. Thorac Cardiovasc Surg 2012;60:398-404. [Crossref] [PubMed]
- Carballo M, Maish MS, Jaroszewski DE, et al. Video-assisted thoracic surgery (VATS) as a safe alternative for the resection of pulmonary metastases: a retrospective cohort study. J Cardiothorac Surg 2009;4:13. [Crossref] [PubMed]
- Gossot D, Radu C, Girard P, et al. Resection of pulmonary metastases from sarcoma: can some patients benefit from a less invasive approach? Ann Thorac Surg 2009;87:238-43. [Crossref] [PubMed]
- Nakajima J, Murakawa T, Fukami T, et al. Is thoracoscopic surgery justified to treat pulmonary metastasis from colorectal cancer? Interact Cardiovasc Thorac Surg 2008;7:212-6. [Crossref] [PubMed]
- Mutsaerts EL, Zoetmulder FA, Meijer S, et al. Long term survival of thoracoscopic metastasectomy vs. metastasectomy by thoracotomy in patients with a solitary pulmonary lesion. Eur J Surg Oncol 2002;28:864-8. [Crossref] [PubMed]
- Watanabe M, Deguchi H, Sato M, et al. Midterm results of thoracoscopic surgery for pulmonary metastases especially from colorectal cancers. J Laparoendosc Adv Surg Tech A 1998;8:195-200. [Crossref] [PubMed]
- Pastorino U, Buyse M, Friedel G, et al. International Registry of Lung Metastases. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg 1997;113:37-49. [Crossref] [PubMed]
- Ost DE, Gould MK. Decision making in patients with pulmonary nodules. Am J Respir Crit Care Med 2012;185:363-72. [Crossref] [PubMed]
- Cheang MY, Herle P, Pradhan N, et al. Video-assisted thoracoscopic surgery versus open thoracotomy for pulmonary metastasectomy: a systematic review. ANZ J Surg 2015;85:408-13. [Crossref] [PubMed]
- Dong S, Zhang L, Li W, et al. Evaluation of video-assisted thoracoscopic surgery for pulmonary metastases: a meta-analysis. PLoS One 2014;9:e85329. [Crossref] [PubMed]
- Greenwood A, West D. Is a thoracotomy rather than thoracoscopic resection associated with improved survival after pulmonary metastasectomy? Interact Cardiovasc Thorac Surg 2013;17:720-4. [Crossref] [PubMed]
- Meng D, Fu L, Wang L, Dai Y, et al. Video-assisted thoracoscopic surgery versus open thoracotomy in pulmonary metastasectomy: a meta-analysis of observational studies. Interact Cardiovasc Thorac Surg 2016;22:200-6. [Crossref] [PubMed]
- Sauvain MO, Abdelnour-Berchtold E, Zellweger M, et al. Why choosing a video-assisted thoracic surgery approach for pulmonary metastasectomy? J Vis Surg 2019;5:46. [Crossref]
- Migliore M, Jakovic R, Hensens A, et al. Extending surgery for pulmonary metastasectomy: what are the limits? J Thorac Oncol 2010;5:S155-60. [Crossref] [PubMed]
- Migliore M, Criscione A, Calvo D, et al. Wider implications of video-assisted thoracic surgery versus open approach for lung metastasectomy. Future Oncol 2015;11:25-29. [Crossref] [PubMed]
Cite this article as: Fiorelli A, Carlucci A, Natale G, Bove M, Freda C, Noro A, Ferrara V, Opromolla G, Martone M, Cascone R, Messina G, Izzo A, Vicidomini G, Santini M. Survival after video-assisted thoracoscopic surgery for lung metastasectomy. Video-assist Thorac Surg 2021;6:2.


