
The clinical application of 18F-FDG PET/CT in pancreatic cancer: a narrative review
Introduction
Pancreatic cancer is a malignant tumor of the digestive system with an inferior prognosis (1). The annual incidence of pancreatic cancer is similar to its annual mortality rate (2). The latest data in 2020 suggest that the 5-year survival rate of patients with pancreatic cancer is still less than 9%, and the number of new cases of pancreatic cancer in males ranks 10th among all malignant tumors and 9th in females (3). However, according to the statistics of the number of deaths caused by tumors, the number of deaths of male and female patients caused by pancreatic cancer ranks fourth among all malignant tumors (3). According to research data released by the National Cancer Center of China, the incidence of pancreatic cancer ranks 8th among malignant tumors in the Chinese urban male population, and its mortality rate ranks 5th among malignant tumors in Beijing and Shanghai (4,5). Pancreatic cancer is often asymptomatic in the early stages, and most patients are in the local or advanced stage at the time of diagnosis and cannot undergo radical surgery (6). To improve the prognosis of patients with pancreatic cancer, it is crucial to diagnose and evaluate pancreatic cancer early (7,8).
Imaging examinations play an essential role in tumor detection, staging, and surgical resection assessment and can provide reliable evidence for the diagnosis and treatment of pancreatic cancer (9). Currently, imaging techniques commonly used for pancreatic cancer include endoscopic ultrasound (EUS), conventional ultrasound, magnetic resonance imaging (MRI), multidetector spiral computed tomography (MDCT), positron emission tomography/computed tomography (PET/CT), and others (10,11). PET/CT is a new imaging device composed of PET and CT (12). 18-Fluorodeoxyglucose (18F-FDG) is a commonly used tracer in the clinic (13). After injection into the body, 18F-FDG-PO4 is generated due to the catalysis of various enzymes, and FDG is not metabolized in the cell (14). Cancer cells are more robust than other ordinary cells in that they can ingest glucose, and the structure of glucose is similar to the structure of 18F-FDG (15). Therefore, after the injection of 18F-FDG, 18F-FDG in tumor cells appears very thick during PET scanning (16). PET imaging can identify tumors in the human body through changes in cellular metabolic levels (17). The combination of PET and CT can determine the metabolic capacity and anatomical position of pancreatic tumor cells in the body and can accurately diagnose the patient's condition and tumor location (18). This article reviews the clinical application of 18F-FDG PET/CT in pancreatic cancer. We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/tcr-21-169).
Diagnostic efficacy in detecting pancreatic cancer
In general, the maximum standardized uptake value (SUVmax) of malignant lesions is high regardless of size, which allows PET/CT to depict small pancreatic lesions (11). The Summary of sensitivity and specificity imaging modality for the diagnosis of PDAC was shown in Table 1. Various studies have reported varying capabilities of PET/CT in the diagnosis of pancreatic cancer (19,20). PET/CT may be considered a first-line imaging examination, but evidence of this method lacks (26). The overall detection sensitivity of PET/CT in the diagnosis of pancreatic cancer is between 90% and 95%, and the specificity is between 82% and 100% (27). A meta-analysis performed in 2011 on 51 studies compared the diagnosis of pancreatic cancer by PET/CT with that by EUS and reported that PET/CT had higher sensitivity (90.1%) and EUS had higher specificity (93.2%). The diagnostic advantage ratio of EUS for pancreatic cancer is significantly higher than that of PET/CT, but its diagnostic value is limited by high heterogeneity between studies (21). Endoscopic ultrasound is very sensitive in the detection of pancreatic lesions with a special resolution of 1–2 mm (28). Studies have demonstrated that EUS, CT and MRI have respective sensitivities of 93, 53 and 67%, respectively, for visualizing tumors 3 cm or smaller. This difference is in fact even more pronounced for lesions smaller than 2 cm (29). And the sensitivity of PET-CT in detecting lesions less than 1 cm in diameter will be significantly reduced to 43% (30). In another meta-analysis performed in 2017, 5,399 patients from 52 studies were included, of which 3,567 had pancreatic cancer. The study found that the sensitivity, specificity, and diagnostic accuracy of PET/CT for pancreatic cancer were 89% (95% CI: 85–93%), 70% (95% CI: 54–84%), and 84% (95% CI: 79–89%), respectively (22).
Table 1
| Author | Year | Study type | Pancreatic cancer–all (n) |
18F-FDG PET-CT | CT | MRI | EUS | References | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sens | Spec | Sens | Spec | Sens | Spec | Sens | Spec | ||||||||
| Sánchez-Bueno F | 2016 | Retrospective study | 139–139 | 0.78 | – | 0.76 | – | – | – | – | – | (19) | |||
| Ergul N | 2014 | Retrospective study | 33–52 | 1 | 0.90 | 0.92 | 0.50 | 0.89 | 0.75 | 1 | 0.88 | (20) | |||
| Tang S | 2009 | Meta-analysis | 3,857* | 0.90 | 0.80 | – | – | – | – | 0.81 | 0.93 | (21) | |||
| Toft J | 2017 | Meta-analysis | 3,567–5,399 | 0.89 | 0.70 | 0.90 | 0.87 | 0.93 | 0.89 | 0.91 | 0.86 | (22) | |||
| Sun Y | 2014 | Retrospective study | 80–91 | 0.68 | 0.73 | – | – | – | – | – | – | (23) | |||
| Ghaneh P | 2018 | Prospective study | 278–583 | 0.93 | 0.76 | 0.89 | 0.71 | – | – | – | – | (24) | |||
| Zhang J | 2015 | Retrospective study | 50–70 | 0.92 | 0.65 | 0.82 | 0.65 | – | – | – | – | (25) | |||
Sens Sensitivity, Spec Specificity. *The study included a total of 3857 patients, but we were unable to obtain the specific number of pancreatic cancer.
Studies have evaluated the role of EUS-guided fine needle aspiration (EUS-FNA) and PET/CT in the preoperative evaluation of pancreatic cancer and found that compared with PET/CT, EUS-FNA has higher sensitivity and accuracy for the preoperative diagnosis of pancreatic cancer. However, PET/CT provides excellent size, volume, and stage information (31). Sun et al. (23) found that the sensitivity, specificity, and accuracy of PET/CT alone in 91 pancreatic cancer patients were 67.5%, 72.73%, and 68.13%, respectively. When combined with the CA19-9 level, these indicators for PET/CT increased to 96.25%, 63.64%, and 92.31%, respectively. The area under the curve (AUC) of the combination of the SUVmax and CA19-9 level was 0.94, which was significantly higher than the AUC of the SUVmax or CA19-9 level alone. However, studies have reported that the negative predictive value of PET/CT in pancreatic lesions suggesting pancreatic cancer is approximately 75% (32). PET/CT negativity does not exclude pancreatic cancer, so a further examination of these PET/CT-negative lesions is necessary. PET/CT can also be used to detect early lesions of pancreatic cancer, pancreatic intraepithelial neoplasia (PanIN). Elevated glucose metabolism has been observed in mouse PanIN and can be detected by PET/CT (33). However, PanIN is an epithelial lesion with a small size. The diagnostic value of FDG-PET for human PanIN is still uncertain, and more clinical studies are needed to verify it.
Staging pancreatic cancer with 18F-FDG PET/CT
In addition to providing significant incremental benefits in the diagnosis of pancreatic cancer, 18F-FDG PET/CT significantly impacts patients’ staging and management with pancreatic cancer (24,25). Heinrich et al. found that PET/CT findings can change the management in 16% of patients with pancreatic cancer deemed resectable after routine staging and were cost-saving (34). Another study reported that among 550 patients with suspected pancreatic cancer, PET/CT correctly changed the stage of 56 pancreatic cancers and affected the treatment of 250 patients. Among the 58 patients preparing for surgery, PET/CT reduced unnecessary surgery by 20% (24). Kim et al. identified 285 patients with early-stage pancreatic cancer who received PET/CT as part of the initial staging workup, and the addition of a PET/CT scan changed the management in 10.9% (n=31) of the 285 patients (35). A meta-analysis performed in 2017 included 1343 patients from 17 clinical studies and showed that PET/CT was more effective than CT in detecting right distant metastases (OR =1.52, 95% CI: 1.23–1.88). However, there was no definite difference between PET/CT and CT in detecting regional lymph node infiltration (OR =0.97, 95% CI: 0.63–1.47). Researchers believe that PET/CT provides a wide range of possibilities for avoiding ineffective radical surgery by detecting occult metastases from pancreatic cancer before surgery. Before developing a surgical plan for patients with pancreatic cancer, surgeons should use PET/CT as a routine preoperative evaluation (36).
Yoneyama et al. suggested that PET/CT could correctly diagnose 88% of lymph node metastases and 91% of distant metastases (37). Wang et al. reported that the optimal SUVmax cutoff value of PET/CT for predicting lymph node micrometastasis was 7.05 (sensitivity: 71.2%, specificity: 76.6%) (38). Besides, metabolic 18F-FDG PET/CT-derived parameters, such as the SUVmax, can be used to predict the venous infiltration status in patients with resectable pancreatic cancer (39). In 2016, the International Pancreatic Society proposed the concept of biological borderline resectable pancreatic ductal adenocarcinoma (BR-PDAC), which was defined as distant or regional lymph node metastases diagnosed by PET/CT and a CA19-9 level >500 U/mL (40). Whether such patients can benefit from immediate surgery remains controversial.
In general, 18F-FDG PET/CT can help improve the detection of occult metastases (Figure 1), ultimately sparing individual patients from potentially unnecessary surgery (Table 2). The diagnostic value of PET/CT for lymph node staging still needs future clinical research.
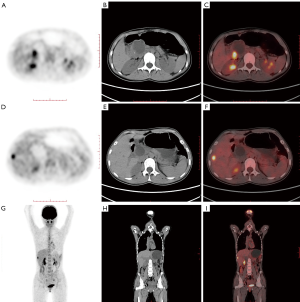
Table 2
| Author | Year | Study type | Pancreatic cancer/all (n) | Sens | Spec | % Change in Management | Description | References |
|---|---|---|---|---|---|---|---|---|
| Ghaneh P | 2018 | Prospective study | 278/583 | 0.93 | 0.76 | 45 | 18F-FDG PET/CT correctly changed the staging of pancreatic cancer in 56 patients and influenced management in 250 (45%) patients. PET/CT stopped resection in 58 (20%) patients who were due to have surgery | (24) |
| Heinrich S | 2005 | Retrospective study | 46/59 | 0.89 | 0.69 | 16 | 18F-FDG PET/CT findings changed the management in 16% of patients with pancreatic cancer deemed resectable after routine staging and was cost saving | (34) |
| Kim R | 2015 | Retrospective study | 285/285 | – | – | 10.9 | 18F-FDG PET/CT helped improve detection of occult metastases, ultimately sparing these patients a potentially unnecessary surgery | (35) |
Sens Sensitivity, Spec Specificity.
Prognostic value of 18F-FDG PET/CT in pancreatic cancer
Pancreatic cancer is a heterogeneous disease with different prognoses in different subgroups (41). In addition to the existing tumor node metastasis (TNM) staging system, several markers can be used to predict the prognosis of patients with pancreatic cancer (42-44). As shown in Table 3, studies have reported that the prognosis of patients with pancreatic cancer can be assessed by PET/CT (45,53). The SUVmax is significantly related to the survival rate of pancreatic cancer patients at each stage, and patients with a low SUV have a longer survival time (46). Zhang et al. found that in patients with locally advanced pancreatic cancer who received stereotactic body radiation therapy (SBRT), the metabolic tumor volume (MTV) and total lesion glycolysis (TLG) detected by PET/CT were independent prognostic factors of overall survival (OS) (47). Choi et al. also found that MTV could provide independent prognostic information on patients with locally advanced pancreatic cancer treated with radiotherapy and chemotherapy. Volume-based PET/CT parameters may help determine which subgroups of patients will benefit from radiation therapy (48). MTV and TLG can also be used to predict the prognosis of patients with pancreatic cancer undergoing surgery. Lee et al. reported 89 patients with pancreatic cancer who underwent surgery, of whom 57 received neoadjuvant chemotherapy, and found that the MTV and TLG were independent predictors of recurrence-free survival (RFS) and OS, regardless of whether the patient received neoadjuvant chemotherapy (49). Another study showed that MTV and TLG were better at predicting OS and RFS than baseline serum CA19-9 levels, the SUVmax, and tumor size (50). MTV and TLG can be used as prognostic indicators for patients with resectable pancreatic cancer (54). Kim et al. retrospectively analyzed the preoperative PET/CT data of 85 patients who underwent radical surgery and found that the SUV ratio (SUV of the lymph node/SUV of the tumor) could predict patients’ prognosis. An SUV ratio greater than 0.384 is an independent risk factor for a poor prognosis (51). In another study, researchers analyzed the prognosis of 40 patients with pancreatic cancer. SUVs were determined at 1 hour (SUV1) and 2 hours (SUV2) after F-FDG injection. The retention index (RI) is defined as the percentage change between the SUV1 and SUV2. The results suggest that an RI of less than 17% shows a significant independent correlation with prolonged survival. The RI is an accurate parameter that predicts the prognosis of pancreatic cancer disease and identifies patients who can benefit from surgery (52). Also, F-FDG INF (global F-FDG influx) was a significant variable for OS in patients with pancreatic cancer (55).
Table 3
| Author | Year | Study type | N | Patients, Treatments | Parameters | Prognostic value | References |
|---|---|---|---|---|---|---|---|
| Hyun S | 2016 | Retrospective study | 137 | Newly diagnosed pancreatic cancer | First-order entropy | Higher entropy (HR, 5.59; P=0.028) was independently associated with worse survival | (45) |
| Hwang J P | 2012 | Retrospective study | 165 | Underwent surgery, radiotherapy, and/or chemotherapy | SUVmax | SUVmax >4.1 (HR, 2.1; P=0.0008) was independently related to OS | (46) |
| Zhang A | 2019 | Retrospective study | 23 | LAPC patients underwent chemo-SBRT combined therapy | MTV | MTV >14.2 cm3 was proved to be the independent prognostic factor for OS (HR, 3.015; P<0.05) | (47) |
| Choi HJ | 2014 | Retrospective study | 60 | LAPC patients underwent chemoradiation therapy | MTV, TLG | MTV >10.0 cm3 and TLG >45.0 g were independent prognostic factors for OS (HR, 2. 21; P=0.038; HR, 2.19; P=0.019) | (48) |
| Lee JW | 2014 | Retrospective study | 87 | Underwent surgical resection | MTV, TLG | MTV >3.0 cm3 and TLG >10.0 g were independent prognostic factors for OS (HR, 3.69; P=0.02; HR, 4.85; P=0.003) and RFS (HR, 2.34; P=0.001; HR, 2.59; P=0.003) | (49) |
| Xu HX | 2014 | Retrospective study | 122 | Underwent radical pancreatectomy | MTV, TLG | MTV >15.7 cm3 and TLG >57.5 g were independent prognostic factors for OS (HR, 1.265; P=0.008; HR, 1.253; P=0.005) and RFS (HR, 1.245; P=0.006; HR, 1.217; P=0.006) | (50) |
| Kim HR | 2018 | Retrospective study | 70 | Underwent radical surgery | Lymph node/tumor SUV ratio | Lymph node/tumor SUV ratio (P=0.007) was independently related to OS | (51) |
| Xi Y | 2014 | Retrospective study | 40 | Underwent surgery and/or chemotherapy. | Retention index | RI less than 17% was significant independent associated with prolonged patient survival (P<0.05) | (52) |
HR, hazard ratio; OS, overall survival; PFS, progression-free survival; LAPC, locally advanced pancreatic cancer; SBRT, stereotactic body radiation therapy; MTV, metabolic tumor volume; TLG, total lesion glycolysis. Retention index: SUVs were determined at 1 h (SUV1) and 2 h (SUV2) after 18F-FDG injection, the retention index was defined as the RI less than 17% percentage change between SUV1 and SUV2.
Overall, many 18F-FDG PET/CT PET/CT parameters can be used to predict the prognosis of pancreatic cancer, but it is still inconclusive as to which index can best predict the prognosis. Multicenter prospective data are needed for verification.
18F-FDG PET/CT for monitoring the treatment effect and detecting recurrent pancreatic cancer
Pancreatic cancer is prone to recurrence after surgery, but a radiographic evaluation after pancreatic tumor resection is challenging, and it is particularly difficult to distinguish between local tumor recurrence and postoperative fibrosis (56). PET/CT can help detect pancreatic tumor recurrence (57,58). A systematic review and meta-analysis in 2017 analyzed the imaging data of 333 patients with pancreatic cancer from 7 studies. The study found that in detecting pancreatic cancer recurrence, the sensitivity and specificity of CT were 0.70 and 0.80, respectively. For FDG PET/CT, the combined sensitivity and specificity estimates were 0.88 and 0.89, respectively. For FDG PET/CT and contrast-enhanced CT, the combined sensitivity and specificity estimates were 0.95 and 0.81, respectively (59). Besides, research reports that PET-CT is significantly more sensitive than CT when detecting distant recurrences, and PET/CT can detect recurrences in areas not covered by CT (57). Therefore, as shown in Figure 2, when CT is negative or ambiguous, PET/CT may have added value if pancreatic cancer is suspected of recurring. Rayamajhi et al. analyzed PET/CT images and the CA19-9 level in 39 patients with pancreatic cancer. The recurrence sensitivity, specificity, and accuracy of PET/CT were 90.9%, 100.0%, and 92.3%, respectively. PET/CT detected recurrence in 12 patients with normal CA19-9 levels. It has been suggested that PET/CT is highly sensitive to the recurrence of pancreatic cancer and that recurrence can be detected in patients with normal CA19-9 levels (60).
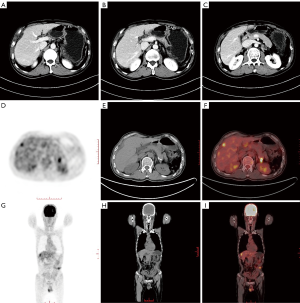
18F-FDG PET/CT can also dynamically monitor the efficacy of treatment (59,61,62).
A meta-analysis in 2019 included 995 patients (683 with borderline resectable pancreatic cancer and 312 with locally advanced pancreatic cancer) receiving neoadjuvant therapy from 15 studies. A comparison of PET/CT images before and after neoadjuvant chemotherapy revealed that the decrease in the SUVmax was positively correlated with resectability, suggesting that a decrease in the tumor SUVmax on PET-CT may be a potential marker of the neoadjuvant chemotherapy response and resectability (63). Michl et al. compared the PET/CT images of 17 patients with liver metastases from pancreatic cancer before and three months after radioembolization, and the results suggested that changes in the SUVpeak and TLG could predict OS, progression-free survival (PFS), and the time to intrahepatic progression after pancreatic cancer liver metastases (64). Yue et al. found that using pre-and postradiotherapy PET/CT images could identify intratumoral heterogeneity in patients with pancreatic cancer and be used to evaluate the clinical results of radiotherapy based on the level of heterogeneity. This technique can also stratify patient risk and help select the appropriate treatment strategy for each patient (65).
18F-FDG PET/CT incidental detection of second primary tumors
When patients undergo whole-body PET/CT, unexpected FDG uptake areas may be found, increasing the possibility of a second primary tumor (66). A second primary tumor was reportedly incidentally found upon PET/CT examinations of patients with head and neck cancer (67), esophageal cancer (68), colorectal cancer (69), and lung cancer (70). Similar reports have been made in patients with the pancreatic disease (Figure 3). Moletta et al. retrospectively analyzed PET/CT images of 399 patients with pancreatic disease. Among them, 31 patients exhibited unexpected focal FDG uptake and were diagnosed with 22 invasive malignancies. Patients in whom a second primary tumor was found incidentally by PET/CT and underwent resection of the tumor experienced prolonged survival (71).
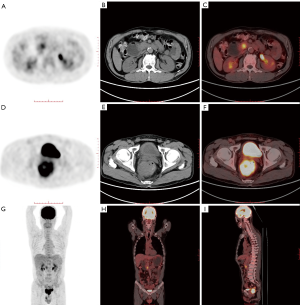
Besides, there have been reports of patients with diseases other than pancreatic cancer found incidentally during PET/CT examinations (72). Sato et al. performed 497 consecutive PET/CT examinations on 290 patients with malignant lymphoma, 8 of whom (2.8%) were pathologically confirmed as having a second primary cancer, including one pancreatic cancer. It is worth noting that PET/CT showed that 5 of the eight patients (62.5%) had a high accumulation of FDG, and there was no corresponding tumor in conventional CT, which facilitated the early detection and successful treatment of the second primary tumor (73).
Limitation of 18F-FDG PET/CT in pancreatic cancer
Although PET/CT plays an essential role in diagnosing, treating, and managing pancreatic cancer, it still has certain limitations. In some cases, it is challenging to distinguish autoimmune pancreatitis (Figure 4) from pancreatic cancer by PET/CT (74-76). Both autoimmune pancreatitis and pancreatic cancer appear as metabolic abnormalities and increased FDG accumulation (77,78). Hsu et al. described a 52-year-old patient with subacute upper abdominal pain. The patient’s CT showed an enlarged pancreatic head with hepatic vascular encapsulation, and PET/CT showed increased accumulation of FDG, which is highly suggestive of pancreatic cancer. After an open biopsy, a morphological examination revealed the pancreas’ inflammatory infiltration, consistent with chronic sclerosing pancreatitis. Further laboratory tests showed elevated serum IgG4 levels, which confirmed the diagnosis of sclerosing pancreatitis (79). Cheng et al. compared the PET/CT scan results of 53 patients with suspected autoimmune pancreatitis and 61 pancreatic cancer patients, and the results suggested that PET/CT is slightly less specific in distinguishing autoimmune pancreatitis from pancreatic cancer (80). Some researchers also believe that PET/CT may help distinguish autoimmune pancreatitis from pancreatic cancer (81-83). Zhang et al. reviewed the FDG PET/CT results of 26 patients with autoimmune pancreatitis and 40 patients with pancreatic cancer and found that the SUVmax between autoimmune pancreatitis and pancreatic cancer was significant in early and delayed PET/CT scans. In contrast, only in patients with autoimmune pancreatitis can the accumulation of diffuse pancreatic FDG and increased uptake of inverted “V” FDG in the prostate be found simultaneously and help identify autoimmune pancreatitis and pancreatic cancer by PET/CT (84).
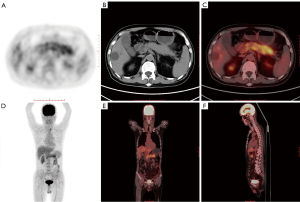
Also, 18F-FDG PET/CT has limited ability to distinguish between nonmetastatic pancreatic cancer and mass pancreatitis (85). Kato analyzed the PET/CT results of 47 patients with pancreatic masses and no metastasis. Among these patients, 33 were eventually diagnosed with pancreatic cancer, and the other 14 were diagnosed with pancreatitis. It was found that there was still a considerable SUVmax between the two diseases. Overlapping and no significant differences in FDG uptake patterns were found in the mass areas, suggesting that it is difficult for PET/CT to distinguish between nonmetastatic pancreatic cancer and mass pancreatitis (86). Ye et al. described a 59-year-old male patient whose PET/CT imaging showed that the border of the pancreatic head's soft tissue mass was unclear, with a maximum SUV of 4.39. Low-density shadows with unclear boundaries were also found in the liver’s left lobe, with a maximum SUV of 4.13, suggesting pancreatic cancer metastasis to the liver. However, postoperative pathology was consistent with chronic pancreatitis, schistosomiasis, and granulomatous liver inflammation (87).
New targets and new PET/CT tracers in pancreatic cancer
Considering that 18F-FDG PET/CT has certain limitations in the diagnosis of pancreatic cancer, new targets and new PET tracers are continually being developed and used (88). Flores et al. developed the first 18F-labeled lactose analog that targets HIP/PAP and applied it to the early detection of pancreatic cancer by PET in an animal model (89). Hausner et al. prepared an αvβ6-binding peptide (αvβ6-BP) and radiolabeled it with 4-18F-fluorobenzoic acid. PET images showed a massive uptake of αvβ6-BP in both the primary and metastatic foci, including metastases to the brain, bone, liver, and lung (90). Besides, (4S)-4-(3-18F-fluoro propyl)-L-glutamate (FSPG) PET reflects system xC- transporter (xCT) expression, has been used to detect pancreatic cancer and improves the detection of liver metastasis (91). Nielsen et al. used F-fluorobenzoate to radioactively label active site-inhibited factor VIIa (FVIIai) for the specific and noninvasive imaging of tissue factors in pancreatic cancer (92).
Zettlitz et al. developed a double-labeled probe based on the A2 cysteine diabody (A2cDb) that targets cell surface prostate stem cell antigen (PSCA) expressed in most pancreatic cancers and supported the dual-mode detection of pro-antigen-specific PET (immuno-PET) and intraoperative near-infrared fluorescence (NIRF). High-contrast immunological PET/NIRF images of pancreatic ductal adenocarcinoma xenografts (PDX-PDAC) can be obtained using dual-mode imaging anti-PSCA cys-dual antibodies, indicating that the imaging agent may also provide noninvasive whole-body imaging to locate PSCA-positive pancreatic cancer and identify tumor edges during fluorescent image-guided surgery (93). Houghton et al. reported the application of PET, NIRF, and dual-modal (PET/NIRF) imaging agents using 5B1, a fully human monoclonal antibody that targets CA19-9, a well-established pancreatic cancer biomarker. Validated by xenograft animal models, this imaging agent has a significant ability to delineate metastases and map sentinel nodes by PET/CT and NIRF imaging (94). Houghton et al. modified 5B1 with trans cyclooctene (TCO) and synthesized a novel NOTA-PEG7-Tz radioligand. They suggested that the 5B1-TCO and (64) Cu-NOTA-PEG7-Tz systems can delineate CA19-9-positive xenografts in murine models of pancreatic cancer (95). Besides, immuno-PET with the radiolabeled high-affinity antibody HuMab-5B1 (MVT-2163) binds to the cancer antigen CA19-9 and can identify the source of elevated biomarkers in patients with pancreatic cancer. Lohrmann et al. injected MVT-2163 into 12 patients with CA19-9-positive metastatic pancreatic cancer and performed four whole-body PET/CT scans within one week. As a result, radiotracer absorption was observed not only in metastases shown by conventional CT but also in lymph nodes just centimeters below specific metastatic sites of pancreatic cancer, suggesting that circulating tumor antigen CA19-9 can be used for the sensitive detection of primary tumors and metastatic diseases by immuno-PET (96).
Besides, Loktev et al. developed an iodine-labeled and DOTA-conjugated radiotracer based on fibroblast activation protein specific enzyme inhibitor (FAPI), and imaged patients with 68Ga-labeled FAPI in 2018 (97,98). Radiolabeled FAPI can be quickly imaged with high contrast in tumors with high proportion of stroma (99,100). Kratochwil et al. performed 68Ga-FAPI PET/CT on 80 patients with 28 different tumors. In pancreatic cancer, the average SUVmax is at a moderate level (SUV 6–12). Due to the low background of muscles and blood pools (SUVmax <2), the contrast of medium-intensity pancreatic cancer to the background is more than 3 times (101). Röhrich et al. performed 68Ga-FAPI-PET/CT imaging on 19 patients with pancreatic cancer. Compared with enhanced CT, 68Ga-FAPI-PET/CT changed the staging of 10 patients (102). These findings significantly broaden the number of molecular targets available for PET imaging.
Conclusions
In general, 18F-FDG PET/CT plays a vital role in early diagnosis, and accurate staging predicts survival and monitors therapeutic effectiveness and pancreatic cancer recurrence. Although 18F-FDG PET/CT has limitations in identifying inflammatory diseases and tumors, it still has good development potential. With the development of various new imaging agents, PET/CT will play a more critical role in the clinical diagnosis and treatment of pancreatic cancer.
Acknowledgments
Thanks to Allison Williams for editing this article.
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://dx.doi.org/10.21037/tcr-21-169
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/tcr-21-169). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kindler HL. A Glimmer of Hope for Pancreatic Cancer. N Engl J Med 2018;379:2463-4. [Crossref] [PubMed]
- Kamisawa T, Wood LD, Itoi T, et al. Pancreatic cancer. Lancet 2016;388:73-85. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- Chen W, Sun K, Zheng R, et al. Cancer incidence and mortality in China, 2014. Chin J Cancer Res 2018;30:1-12. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Strobel O, Neoptolemos J, Jäger D, et al. Optimizing the outcomes of pancreatic cancer surgery. Nat Rev Clin Oncol 2019;16:11-26. [Crossref] [PubMed]
- Zhou B, Xu JW, Cheng YG, et al. Early detection of pancreatic cancer: Where are we now and where are we going? Int J Cancer 2017;141:231-41. [Crossref] [PubMed]
- Kikuyama M, Kamisawa T, Kuruma S, et al. Early Diagnosis to Improve the Poor Prognosis of Pancreatic Cancer. Cancers (Basel) 2018;10:48. [Crossref] [PubMed]
- Horvat N, Ryan DE, LaGratta MD, et al. Imaging for pancreatic ductal adenocarcinoma. Chin Clin Oncol 2017;6:62. [Crossref] [PubMed]
- Burk KS, Lo GC, Gee MS, et al. Imaging and Screening of Pancreatic Cancer. Radiol Clin North Am 2017;55:1223-34. [Crossref] [PubMed]
- Nunna P, Sheikhbahaei S, Ahn S, et al. The Role of Positron Emission Tomography/Computed Tomography in Management and Prediction of Survival in Pancreatic Cancer. J Comput Assist Tomogr 2016;40:142-51. [Crossref] [PubMed]
- Debebe SA, Goryawala M, Adjouadi M, et al. 18F-FLT Positron Emission Tomography/Computed Tomography Imaging in Pancreatic Cancer: Determination of Tumor Proliferative Activity and Comparison with Glycolytic Activity as Measured by 18F-FDG Positron Emission Tomography/Computed Tomography Imaging. Mol Imaging Radionucl Ther 2016;25:32-8. [Crossref] [PubMed]
- Basu S, Alavi A. SPECT-CT and PET-CT in Oncology - An Overview. Curr Med Imaging Rev 2011;7:202-9. [Crossref]
- Groheux D, Cochet A, Humbert O, et al. 18F-FDG PET/CT for Staging and Restaging of Breast Cancer. J Nucl Med 2016;57:17S-26S. [Crossref] [PubMed]
- Liberti MV, Locasale JW. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem Sci 2016;41:211-8. [Crossref] [PubMed]
- Jadvar H. PET of Glucose Metabolism and Cellular Proliferation in Prostate Cancer. J Nucl Med 2016;57:25S-9S. [Crossref] [PubMed]
- Delbeke D, Martin WH. PET and PET/CT for Pancreatic Malignancies. PET Clin 2008;3:155-67. [Crossref] [PubMed]
- Dibble EH, Karantanis D, Mercier G, et al. PET/CT of cancer patients: part 1, pancreatic neoplasms. AJR Am J Roentgenol 2012;199:952-67. [Crossref] [PubMed]
- Sánchez-Bueno F, García-Pérez R, Claver Valderas MA, et al. Utility of 18 fludeoxyglucose in preoperative positon-emission tomography-computed tomography (PET-CT) in the early diagnosis of exocrine pancreatic cancer: A study of 139 resected cases. Cir Esp 2016;94:511-7. [PubMed]
- Ergul N, Gundogan C, Tozlu M, et al. Role of (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in diagnosis and management of pancreatic cancer; comparison with multidetector row computed tomography, magnetic resonance imaging and endoscopic ultrasonography. Rev Esp Med Nucl Imagen Mol 2014;33:159-64. [Crossref] [PubMed]
- Tang S, Huang G, Liu J, et al. Usefulness of 18F-FDG PET, combined FDG-PET/CT and EUS in diagnosing primary pancreatic carcinoma: a meta-analysis. Eur J Radiol 2011;78:142-50. [Crossref] [PubMed]
- Toft J, Hadden WJ, Laurence JM, et al. Imaging modalities in the diagnosis of pancreatic adenocarcinoma: A systematic review and meta-analysis of sensitivity, specificity and diagnostic accuracy. Eur J Radiol 2017;92:17-23. [Crossref] [PubMed]
- Sun Y, Duan Q, Wang S, et al. Diagnosis of pancreatic cancer using 18F-FDG PET/CT and CA19-9 with SUVmax association to clinical characteristics. J BUON 2015;20:452-9. [PubMed]
- Ghaneh P, Hanson R, Titman A, et al. PET-PANC: multicentre prospective diagnostic accuracy and health economic analysis study of the impact of combined modality 18fluorine-2-fluoro-2-deoxy-d-glucose positron emission tomography with computed tomography scanning in the diagnosis and management of pancreatic cancer. Health Technol Assess 2018;22:1-114. [Crossref] [PubMed]
- Zhang J, Zuo CJ, Jia NY, et al. Cross-modality PET/CT and contrast-enhanced CT imaging for pancreatic cancer. World J Gastroenterol 2015;21:2988-96. [Crossref] [PubMed]
- Kadhim LA, Dholakia AS, Herman JM, et al. The role of 18F-fluorodeoxyglucose positron emission tomography in the management of patients with pancreatic adenocarcinoma. J Radiat Oncol 2013;2:341-52. [Crossref] [PubMed]
- Pakzad F, Groves AM, Ell PJ. The role of positron emission tomography in the management of pancreatic cancer. Semin Nucl Med 2006;36:248-56. [Crossref] [PubMed]
- Munroe CA, Fehmi SM, Savides TJ. Endoscopic ultrasound in the diagnosis of pancreatic cancer. Expert Opin Med Diagn 2013;7:25-35. [Crossref] [PubMed]
- Müller MF, Meyenberger C, Bertschinger P, et al. Pancreatic tumors: evaluation with endoscopic US, CT, and MR imaging. Radiology 1994;190:745-51. [Crossref] [PubMed]
- Nguyen AH, Melstrom LG. Use of imaging as staging and surgical planning for pancreatic surgery. Hepatobiliary Surg Nutr 2020;9:603-14. [Crossref] [PubMed]
- Sur YK, Kim YC, Kim JK, et al. Comparison of Ultrasound-Guided Core Needle Biopsy and Endoscopic Ultrasound-Guided Fine-Needle Aspiration for Solid Pancreatic Lesions. J Ultrasound Med 2015;34:2163-9. [Crossref] [PubMed]
- Lin JL, Barthel JS, Keshishian J, et al. Negative predictive value of positron emission tomography/computed tomography in patients with a clinical suspicion of pancreatic cancer. Pancreas 2011;40:653-6. [Crossref] [PubMed]
- Fendrich V, Schneider R, Maitra A, et al. Detection of precursor lesions of pancreatic adenocarcinoma in PET-CT in a genetically engineered mouse model of pancreatic cancer. Neoplasia 2011;13:180-6. [Crossref] [PubMed]
- Heinrich S, Goerres GW, Schäfer M, et al. Positron emission tomography/computed tomography influences on the management of resectable pancreatic cancer and its cost-effectiveness. Ann Surg 2005;242:235-43. [Crossref] [PubMed]
- Kim R, Prithviraj G, Kothari N, et al. PET/CT Fusion Scan Prevents Futile Laparotomy in Early Stage Pancreatic Cancer. Clin Nucl Med 2015;40:e501-5. [Crossref] [PubMed]
- Wang L, Dong P, Wang WG, et al. Positron emission tomography modalities prevent futile radical resection of pancreatic cancer: A meta-analysis. Int J Surg 2017;46:119-25. [Crossref] [PubMed]
- Yoneyama T, Tateishi U, Endo I, et al. Staging accuracy of pancreatic cancer: comparison between non-contrast-enhanced and contrast-enhanced PET/CT. Eur J Radiol 2014;83:1734-9. [Crossref] [PubMed]
- Wang S, Shi H, Yang F, et al. The value of 18F-FDG PET/CT and carbohydrate antigen 19-9 in predicting lymph node micrometastases of pancreatic cancer. Abdom Radiol (NY) 2019;44:4057-62. [Crossref] [PubMed]
- Myssayev A, Myssayev A, Ideguchi R, et al. Usefulness of FDG PET/CT derived parameters in prediction of histopathological finding during the surgery in patients with pancreatic adenocarcinoma. PLoS One 2019;14:e0210178. [Crossref] [PubMed]
- Isaji S, Mizuno S, Windsor JA, et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology 2018;18:2-11. [Crossref] [PubMed]
- Sidaway P. Pancreatic cancer: TCGA data reveal a highly heterogeneous disease. Nat Rev Clin Oncol 2017;14:648. [PubMed]
- Fahrmann JF, Bantis LE, Capello M, et al. A Plasma-Derived Protein-Metabolite Multiplexed Panel for Early-Stage Pancreatic Cancer. J Natl Cancer Inst 2019;111:372-9. [Crossref] [PubMed]
- Luo G, Liu C, Guo M, et al. Potential Biomarkers in Lewis Negative Patients With Pancreatic Cancer. Ann Surg 2017;265:800-5. [Crossref] [PubMed]
- Nahm CB, Turchini J, Jamieson N, et al. Biomarker panel predicts survival after resection in pancreatic ductal adenocarcinoma: A multi-institutional cohort study. Eur J Surg Oncol 2019;45:218-24. [Crossref] [PubMed]
- Hyun SH, Kim HS, Choi SH, et al. Intratumoral heterogeneity of (18)F-FDG uptake predicts survival in patients with pancreatic ductal adenocarcinoma. Eur J Nucl Med Mol Imaging 2016;43:1461-8. [Crossref] [PubMed]
- Hwang JP, Lim I, Chang KJ, et al. Prognostic value of SUVmax measured by Fluorine-18 Fluorodeoxyglucose Positron Emission Tomography with Computed Tomography in Patients with Pancreatic Cancer. Nucl Med Mol Imaging 2012;46:207-14. [Crossref] [PubMed]
- Zhang A, Ren S, Yuan Y, et al. Prognostic values of 18F-FDG PET/CT metabolic parameters and clinical figures in locally advanced pancreatic cancer underwent chemotherapy combined with stereotactic body radiation therapy. Medicine (Baltimore) 2019;98:e15064. [Crossref] [PubMed]
- Choi HJ, Lee JW, Kang B, et al. Prognostic significance of volume-based FDG PET/CT parameters in patients with locally advanced pancreatic cancer treated with chemoradiation therapy. Yonsei Med J 2014;55:1498-506. [Crossref] [PubMed]
- Lee JW, Kang CM, Choi HJ, et al. Prognostic Value of Metabolic Tumor Volume and Total Lesion Glycolysis on Preoperative 18F-FDG PET/CT in Patients with Pancreatic Cancer. J Nucl Med 2014;55:898-904. [Crossref] [PubMed]
- Xu HX, Chen T, Wang WQ, et al. Metabolic tumour burden assessed by 18F-FDG PET/CT associated with serum CA19-9 predicts pancreatic cancer outcome after resection. Eur J Nucl Med Mol Imaging 2014;41:1093-102. [Crossref] [PubMed]
- Kim HR, Seo M, Nah YW, et al. Clinical impact of fluorine-18-fluorodeoxyglucose positron emission tomography/computed tomography in patients with resectable pancreatic cancer: diagnosing lymph node metastasis and predicting survival. Nucl Med Commun 2018;39:691-8. [Crossref] [PubMed]
- Xi Y, Guo R, Hu J, et al. 18F-fluoro-2-deoxy-D-glucose retention index as a prognostic parameter in patients with pancreatic cancer. Nucl Med Commun 2014;35:1112-8. [Crossref] [PubMed]
- Parikh U, Marcus C, Sarangi R, et al. FDG PET/CT in Pancreatic and Hepatobiliary Carcinomas: Value to Patient Management and Patient Outcomes. PET Clin 2015;10:327-43. [Crossref] [PubMed]
- Kim YI, Kim YJ, Paeng JC, et al. Heterogeneity index evaluated by slope of linear regression on 18F-FDG PET/CT as a prognostic marker for predicting tumor recurrence in pancreatic ductal adenocarcinoma. Eur J Nucl Med Mol Imaging 2017;44:1995-2003. [Crossref] [PubMed]
- Epelbaum R, Frenkel A, Haddad R, et al. Tumor aggressiveness and patient outcome in cancer of the pancreas assessed by dynamic 18F-FDG PET/CT. J Nucl Med 2013;54:12-8. [Crossref] [PubMed]
- Javadi S, Karbasian N, Bhosale P, et al. Imaging findings of recurrent pancreatic cancer following resection. Abdom Radiol (NY) 2018;43:489-96. [Crossref] [PubMed]
- Jung W, Jang JY, Kang MJ, et al. The clinical usefulness of 18F-fluorodeoxyglucose positron emission tomography-computed tomography (PET-CT) in follow-up of curatively resected pancreatic cancer patients. HPB (Oxford) 2016;18:57-64. [Crossref] [PubMed]
- Kitajima K, Murakami K, Yamasaki E, et al. Performance of integrated FDG-PET/contrast-enhanced CT in the diagnosis of recurrent pancreatic cancer: comparison with integrated FDG-PET/non-contrast-enhanced CT and enhanced CT. Mol Imaging Biol 2010;12:452-9. [Crossref] [PubMed]
- Daamen LA, Groot VP, Goense L, et al. The diagnostic performance of CT versus FDG PET-CT for the detection of recurrent pancreatic cancer: a systematic review and meta-analysis. Eur J Radiol 2018;106:128-36. [Crossref] [PubMed]
- Rayamajhi S, Balachandran A, Katz M, et al. Utility of (18) F-FDG PET/CT and CECT in conjunction with serum CA 19-9 for detecting recurrent pancreatic adenocarcinoma. Abdom Radiol (NY) 2018;43:505-13. [Crossref] [PubMed]
- Shi S, Ji S, Qin Y, et al. Metabolic tumor burden is associated with major oncogenomic alterations and serum tumor markers in patients with resected pancreatic cancer. Cancer Lett 2015;360:227-33. [Crossref] [PubMed]
- Nielsen CH, Jensen MM, Kristensen LK, et al. In vivo imaging of therapy response to a novel pan-HER antibody mixture using FDG and FLT positron emission tomography. Oncotarget 2015;6:37486-99. [Crossref] [PubMed]
- Barreto SG, Loveday B, Windsor JA, et al. Detecting tumour response and predicting resectability after neoadjuvant therapy for borderline resectable and locally advanced pancreatic cancer. ANZ J Surg 2019;89:481-7. [Crossref] [PubMed]
- Michl M, Lehner S, Paprottka PM, et al. Use of PERCIST for Prediction of Progression-Free and Overall Survival After Radioembolization for Liver Metastases from Pancreatic Cancer. J Nucl Med 2016;57:355-60. [Crossref] [PubMed]
- Yue Y, Osipov A, Fraass B, et al. Identifying prognostic intratumor heterogeneity using pre- and post-radiotherapy 18F-FDG PET images for pancreatic cancer patients. J Gastrointest Oncol 2017;8:127-38. [Crossref] [PubMed]
- Beatty JS, Williams HT, Aldridge BA, et al. Incidental PET/CT findings in the cancer patient: how should they be managed? Surgery 2009;146:274-81. [Crossref] [PubMed]
- Haerle SK, Strobel K, Hany TF, et al. (18)F-FDG-PET/CT versus panendoscopy for the detection of synchronous second primary tumors in patients with head and neck squamous cell carcinoma. Head Neck 2010;32:319-25. [PubMed]
- Adams HL, Jaunoo SS. Clinical significance of incidental findings on staging positron emission tomography for oesophagogastric malignancies. Ann R Coll Surg Engl 2014;96:207-10. [Crossref] [PubMed]
- Ladrón-de-Guevara D, Pérez D, Núñez P, et al. Synchronous tumors detected with contrast-enhanced F18-FDG positron emission tomography/computed tomography (PET/CTc) in colorectal cancer. Rev Med Chil 2019;147:828-35. [PubMed]
- Chopra A, Ford A, De Noronha R, et al. Incidental findings on positron emission tomography/CT scans performed in the investigation of lung cancer. Br J Radiol 2012;85:e229-37. [Crossref] [PubMed]
- Moletta L, Bissoli S, Fantin A, et al. PET/CT incidental detection of second tumor in patients investigated for pancreatic neoplasms. BMC Cancer 2018;18:531. [Crossref] [PubMed]
- Kulkarni M, Purandare N, Zade A, et al. FDG PET/CT detects clinically occult pancreatic cancer in a case of Von Hippel-Lindau syndrome. Clin Nucl Med 2013;38:e302-3. [Crossref] [PubMed]
- Sato K, Ozaki K, Fujiwara S, et al. Incidental carcinomas detected by PET/CT scans in patients with malignant lymphoma. Int J Hematol 2010;92:647-50. [Crossref] [PubMed]
- Li G, Liu T, Zheng J, et al. Untypical autoimmune pancreatitis and pancreatic cancer: differential diagnosis experiences extracted from misdiagnose of two cases. Orphanet J Rare Dis 2019;14:245. [Crossref] [PubMed]
- Cao Z, Tian R, Zhang T, et al. Localized Autoimmune Pancreatitis: Report of a Case Clinically Mimicking Pancreatic Cancer and a Literature Review. Medicine (Baltimore) 2015;94:e1656. [Crossref] [PubMed]
- Han L, Jiang L, Tan H, et al. An atypical case of IgG4-related sclerosing pancreatitis on PET/CT imaging. Clin Nucl Med 2014;39:e236-8. [Crossref] [PubMed]
- Nanni C, Romagnoli R, Rambaldi I, et al. FDG PET/CT in autoimmune pancreatitis. Eur J Nucl Med Mol Imaging 2014;41:1264-5. [Crossref] [PubMed]
- Zheng L, Xing H, Li F, et al. Focal Autoimmune Pancreatitis Mimicking Pancreatic Cancer on FDG PET/CT Imaging. Clin Nucl Med 2018;43:57-9. [Crossref] [PubMed]
- Hsu WL, Chang SM, Wu PY, et al. Localized autoimmune pancreatitis mimicking pancreatic cancer: Case report and literature review. J Int Med Res 2018;46:1657-65. [Crossref] [PubMed]
- Cheng MF, Guo YL, Yen RF, et al. Clinical Utility of FDG PET/CT in Patients with Autoimmune Pancreatitis: a Case-Control Study. Sci Rep 2018;8:3651. [Crossref] [PubMed]
- Lee TY, Kim MH, Park DH, et al. Utility of 18F-FDG PET/CT for differentiation of autoimmune pancreatitis with atypical pancreatic imaging findings from pancreatic cancer. AJR Am J Roentgenol 2009;193:343-8. [Crossref] [PubMed]
- Zhao Z, Wang Y, Guan Z, et al. Utility of FDG-PET/CT in the diagnosis of IgG4-related diseases. Clin Exp Rheumatol 2016;34:119-25. [PubMed]
- Santhosh S, Bhattacharya A, Harisankar CN, et al. Role of 18F-FDG PET/CT in the management of a case of autoimmune pancreatitis with extrapancreatic manifestations. Clin Nucl Med 2013;38:e423-5. [Crossref] [PubMed]
- Zhang J, Jia G, Zuo C, et al. 18F- FDG PET/CT helps differentiate autoimmune pancreatitis from pancreatic cancer. BMC Cancer 2017;17:695. [Crossref] [PubMed]
- Hennessy G, Vamadevan S, Loh H, et al. Pancreatic Cancer Complicated by Pancreatitis Demonstrated on FDG PET/CT. Clin Nucl Med 2017;42:239-40. [Crossref] [PubMed]
- Kato K, Nihashi T, Ikeda M, et al. Limited efficacy of (18)F-FDG PET/CT for differentiation between metastasis-free pancreatic cancer and mass-forming pancreatitis. Clin Nucl Med 2013;38:417-21. [Crossref] [PubMed]
- Ye S, Wang WL, Zhao K. F-18 FDG hypermetabolism in mass-forming focal pancreatitis and old hepatic schistosomiasis with granulomatous inflammation misdiagnosed by PET/CT imaging. Int J Clin Exp Pathol 2014;7:6339-44. [PubMed]
- Alauddin MM, De Palatis L. Current and Future Trends in Early Detection of Pancreatic Cancer: Molecular Targets and PET Probes. Curr Med Chem 2015;22:3370-89. [Crossref] [PubMed]
- Flores LG, Bertolini S, Yeh HH, et al. Detection of pancreatic carcinomas by imaging lactose-binding protein expression in peritumoral pancreas using 18Ffluoroethyl-deoxylactose PET/CT. PLoS One 2009;4:e7977. [Crossref] [PubMed]
- Hausner SH, Bold RJ, Cheuy LY, et al. Preclinical Development and First-in-Human Imaging of the Integrin αvβ6 with 18Fαvβ6-Binding Peptide in Metastatic Carcinoma. Clin Cancer Res 2019;25:1206-15. [Crossref] [PubMed]
- Cheng MF, Huang YY, Ho BY, et al. Prospective comparison of (4S)-4-(3-18F-fluoropropyl)-L-glutamate versus 18F-fluorodeoxyglucose PET/CT for detecting metastases from pancreatic ductal adenocarcinoma: a proof-of-concept study. Eur J Nucl Med Mol Imaging 2019;46:810-20. [Crossref] [PubMed]
- Nielsen CH, Erlandsson M, Jeppesen TE, et al. Quantitative PET Imaging of Tissue Factor Expression Using 18F-Labeled Active Site-Inhibited Factor VII. J Nucl Med 2016;57:89-95. [Crossref] [PubMed]
- Zettlitz KA, Tsai WK, Knowles SM, et al. Dual-Modality Immuno-PET and Near-Infrared Fluorescence Imaging of Pancreatic Cancer Using an Anti-Prostate Stem Cell Antigen Cys-Diabody. J Nucl Med 2018;59:1398-405. [Crossref] [PubMed]
- Houghton JL, Zeglis BM, Abdel-Atti D, et al. Site-specifically labeled CA19.9-targeted immunoconjugates for the PET, NIRF, and multimodal PET/NIRF imaging of pancreatic cancer. Proc Natl Acad Sci U S A 2015;112:15850-5. [Crossref] [PubMed]
- Houghton JL, Zeglis BM, Abdel-Atti D, et al. Pretargeted Immuno-PET of Pancreatic Cancer: Overcoming Circulating Antigen and Internalized Antibody to Reduce Radiation Doses. J Nucl Med 2016;57:453-9. [Crossref] [PubMed]
- Lohrmann C, O'Reilly EM, O'Donoghue JA, et al. Retooling a Blood-Based Biomarker: Phase I Assessment of the High-Affinity CA19-9 Antibody HuMab-5B1 for Immuno-PET Imaging of Pancreatic Cancer. Clin Cancer Res 2019;25:7014-23. [Crossref] [PubMed]
- Loktev A, Lindner T, Mier W, et al. A Tumor-Imaging Method Targeting Cancer-Associated Fibroblasts. J Nucl Med 2018;59:1423-9. [Crossref] [PubMed]
- Lindner T, Loktev A, Altmann A, et al. Development of Quinoline-Based Theranostic Ligands for the Targeting of Fibroblast Activation Protein. J Nucl Med 2018;59:1415-22. [Crossref] [PubMed]
- Loktev A, Lindner T, Burger EM, et al. Development of Fibroblast Activation Protein-Targeted Radiotracers with Improved Tumor Retention. J Nucl Med 2019;60:1421-9. [Crossref] [PubMed]
- Giesel FL, Kratochwil C, Lindner T, et al. 68Ga-FAPI PET/CT: Biodistribution and Preliminary Dosimetry Estimate of 2 DOTA-Containing FAP-Targeting Agents in Patients with Various Cancers. J Nucl Med 2019;60:386-92. [Crossref] [PubMed]
- Kratochwil C, Flechsig P, Lindner T, et al. 68Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J Nucl Med 2019;60:801-5. [Crossref] [PubMed]
- Röhrich M, Naumann P, Giesel FL, et al. Impact of 68Ga-FAPI PET/CT Imaging on the Therapeutic Management of Primary and Recurrent Pancreatic Ductal Adenocarcinomas. J Nucl Med 2021;62:779-86. [Crossref] [PubMed]


