
Bostrycin inhibits growth of tongue squamous cell carcinoma cells by inducing mitochondrial apoptosis
Introduction
Oral cancer is one of the most common malignant tumors in head and neck, and its incidence is increasing year by year in the world (1). Among them, tongue squamous cell carcinoma is the most typical and common type of oral cancer, while 90% of oral cancer is squamous cell carcinoma, in which tongue squamous cell carcinoma is as high as 50–60% (2). There are about 10,990 new cases of tongue squamous cell carcinoma in the world every year (3). Although the medical technology has made great progress, such as surgery, chemotherapy, radiotherapy and other treatments that have been used to treat tongue squamous cell carcinoma, the prognosis of patients with tongue squamous cell carcinoma is still unsatisfactory. The 5-year survival rate of it is only about 50%, and local or regional recurrence and cervical lymph node metastasis remain a huge challenge for clinical treatment (4). Most studies have shown that tongue squamous cell carcinoma cells are highly invasive and prone to invasion and metastasis, which is related to the activation of Wnt/beta-catenin signaling pathway.
Bostrycin was first discovered by Noda in 1986 as a natural pigment product with anthraquinone skeleton, which has great advantages in antimicrobial, antitumor and other aspects (5). bostrycin were found in marine endophytes in most of the studies (6). However, bostrycin isolated from Myceliun sphaeroides of Artemisia argyi has not been reported. In this study, the endophytic fungi HCH285 subordinated to Nigrospora oryzae were isolated from Chinese genuine medicinal herb Artemisia argyi, and the bostrycin were separated from the red pigment in the secondary metabolite from HCH285.
Some studies have shown that bostrycin has antioxidant, antimicrobial and anticancer activities in vitro, but the mechanism is not yet clear. At present, the inhibitory effect of bostrycin on tongue squamous cell carcinoma has not been reported and also the mechanism of it is unclear. This study revealed that both SCC9 and SCC25 cells were inhibited by Bostrycin, which could effectively repress the proliferation by promoting apoptosis of tongue squamous cell carcinoma cells.
Methods
Cell lines and culture conditions
Two tongue squamous cell carcinoma cell lines, SCC9 and SCC25 purchased from American Type Culture Collection (ATCC), were cultured in Dulbecco’s Modified Eagle’S Medium Nutrient Mixture F-12 Ham (DME/F12) medium, supplemented with 400 µg/L hydrocortisone, 60 mg/L sodium pyruvate and 1.2 g/L NaHCO3. 10% fetal bovine serum (BBI) was added to the medium and cultured in incubator with 5% CO2.
Reagent preparation of Bostrycin
Thirty-two mg of bostrycin monomer was dissolved in 1 mL Dimethyl sulfoxide (DMSO) as storage solution. 100 mL of storage solution was then added into 900 mL of sterile ddH2O and 3.2 mg/mL bostrycin was obtained. Sterile water gradient was used to dilute it to 1.6, 0.8, 0.4, 0.2, 0.1 and 0.05 mg/mL. The final concentration of bostrycin monomer in cell cultural medium was 32, 16, 8, 4, 2, 1 and 0.5 µg/mL, respectively.
Cell proliferation assays
SCC9 and SCC25 cells were inoculated into 96-well plates with density of 5,000 cells per hole. After 12 hours, different concentrations of bostrycin were added. After 24, 48 and 72 hours of treatment, 20 µL Thiazolyl Blue Tetrazolium Bromide (MTT) solution (5 mg/mL) was mixed into each hole. After 4 hours culturing, the supernatant was discarded and 150 µL DMSO was added into each hole to dissolve methylic acid. The absorbance was measured at 490 nm by microplate reader after the complete dissolution (7).
Colony formation experiment: 1,000 cells were inoculated in 35 mm dish, then added with bostrycin 12 hours later. The supernatant was discarded, after 24 hours of treatment. Seven days later, methanol/acetic acid was used to fix at room temperature for 30 minutes and 2% crystal violet was stained for 1 hour. Photographs were taken after the dishes were cleaned by PBS three times and colony were counted with Image J software (8).
Cell cycle analysis
Bostrycin were added into 35 mm dishes after SCC9 and SCC25 cultured over night with the confluence degree 70%. Then cells were collected with pre-cooled 70% ethanol for fixing overnight after 24 h treatment. Then cells were washed with phosphate buffered saline (PBS), and added with dyeing solution containing PI, Triton-100 and RNase, analyzed by flow cytometry (BD C6) (9).
Wound-healing assay
SCC9 and SCC25 cells were inoculated in 24-well plate until cell density was over 80–90% after 12 hours. Two vertical lines were drawn in cell layer, and the medium was replaced with medium including 1% serum and different concentrations of Bostrycin. Cells were photographed at the same place every 12 hours. Image J was used to count scratch area and calculate statistical migration rate (10,11).
Annexin V/PI apoptosis analysis
Bostrycin was added into 6 cm dishes when SCC9 and SCC25 cells reach 70% convergence. After 24 hours treatment, cells were collected and stained with Annexin V-Alexa Fluor 488/PI apoptotic kit (Yeasen Biotech, Shanghai, China). Flow cytometry (BD C6) was used to detect apoptosis (12).
AO/EB staining analysis
SCC9 and SCC25 cells were inoculated in 12-well plates, and then bostrycin was added after cultured overnight until 70% confluence. 24 or 48 hours later, acrid orange (AO) and ethidium bromide (EB) dyeing solution were added into dishes with final concentration of 2 µg/mL at room temperature in 2−5 minutes after medium abandoning. Photos were taken by Fluorescence microscopy. Suspensing cells in dyeing solution were analysis with flow cytometry (13).
Mitochondrial apoptosis analysis
SCC9 and SCC25 cells were inoculated in 24 well plate until cell density was about 70%. Cells were treated with different concentrations bostrycin for 24 h, and the positive control was treated with Carbonyl cyanide 3-chlorophenyl hydrazone (CCCP) for 20 mins. Cells were stained with JC-1 after collected, and analysis by flow cytometry after incubated in 37 °C for 20 mins (14,15).
Western blot
SCC9 and SCC25 cells were cultured in 35 mm dishes with 70% confluence. After 24 hours of treatment with Bostrycin, cells were collected to extract the total protein. After polyacrylamide gel electrophoresis, protein samples were transferred to Nitrocellulose (NC) membrane. Then NC membrane was washed by Tris-buffered saline and Tween-20 buffer solution (TBST) several times and incubated in first antibody solution overnight. It was incubated in the second antibody solution for 1 h after cleaning the membrane with TBST, finally for development (16).
Statistical analysis
All values were expressed as mean +standard error. To compare the difference between the two groups, the double-tailed T test was followed by ANOVA. P<0.05 was significant difference.
Results
The proliferation of tongue squamous cell carcinoma cells was inhibited bostrycin
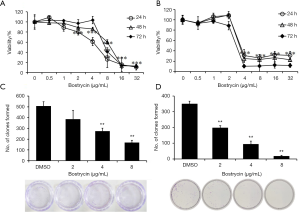
SCC9 and SCC25 cells were treated with different concentrations of bostrycin for 24, 48 and 72 hours, respectively. The viability of SCC9 cells was significantly decreased when the concentration of bostrycin was 4–8 µg/mL (Figure 1A). The IC50 of bostrycin was 5.37 µg/mL to SCC9 cells at 24 h. The viability of SCC25 cells was also significantly reduced when the bostrycin concentration was 2−4 µg/mL, and IC50 was 3.50 µg/mL at 24 h (Figure 1B). With the prolongation of treatment time, the viability of both cells changed slightly in the same concentration of Bostrycin. The results of colony formation experiment showed that the proliferation of SCC9 cells was significantly inhibited when the concentration of bostrycin was greater than or equal to 4 µg/mL, and the inhibition rate of colony formation of SCC9 cells was 45.8% at 4 µg/mL, 66.9% at 8 µg/mL (Figure 1C). The proliferation of SCC25 cells were also suppressed when the concentration of bostrycin was greater than or equal to 2 µg/mL, and the inhibition rate of colony formation of SCC25 cells 43.7% at 2 µg/mL, 73.3% at 4 µg/mL and 95.0% at 8 µg/mL (Figure 1D).
Inhibitory effect of bostrycin on wound-healing of tongue squamous cell carcinoma cells
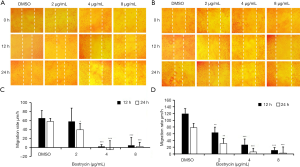
Wound-healing assay showed that the migration of SCC9 and SCC25 cells could be inhibited by bostrycin (Figure 2). At 12 h, the migration rate of SCC9 cells weas decreased by 10.8% at 2 µg/mL of Bostrycin, and that of SCC25 cells weas decreased by 47.1%; 4 µg/mL of bostrycin could inhibit the migration rate of SCC9 cells to 96.5%, and that of SCC25 to 77.1%, respectively. At 24 h, the migration rates of SCC9 and SCC25 treating with 2 µg/mL of bostrycin were decreased by 32.3% and 60.4%, respectively; and that of SCC9 and SCC25 were reduced by 108.1% and 92.3% respectively, when 4 µg/mL of bostrycin existing. The migration rates of SCC9 and SCC25 cells were on the decreased of 95.5% and 96.6% at the concentration of 8 µg/mL, respectively. At the concentration of 2 µg/mL, bostrycin could effectively reduce the migration rate of both tongue squamous cell carcinoma cells, and especially SCC25 cells weas more sensitive. With the increase of concentration, SCC9 and SCC25 were both extremely significant inhibited on account of close to the IC50 of Bostrycin.
The G2/M phase of SCC9 and SCC25 tongue squamous cell carcinoma cells were arrested by Bostrycin
As shown in Figure 3, the sub G1 phase of SCC9 and SCC25 cells were increased by 2.0–2.4% at 2 µg/mL, and that of both cells were raised between 6.5% to 10.1% when the concentration greater than or equal to 4 µg/mL, after treatment with bostrycin for 24 hours. The G0/G1 phase of SCC9 and SCC25 dealing with bostrycin were decreased by about 13.2% and 16.7%, respectively. Interestingly, G2/M phase of SCC9 were increased by 1.9% to 8.8% with the raise of Bostrycin, and that of SCC25 also were increased by 2.7% to 11.7% (Figure 3B,D). The results showed that sub-G1 phase and G2/M phase were increased with the increasing of bostrycin concentration, suggesting that bostrycin could inhibit the cell proliferation by blocking G2/M phase while induce the apoptosis of tongue squamous cell carcinoma cells.

Bostrycin induced the apoptosis of tongue squamous cell carcinoma cells
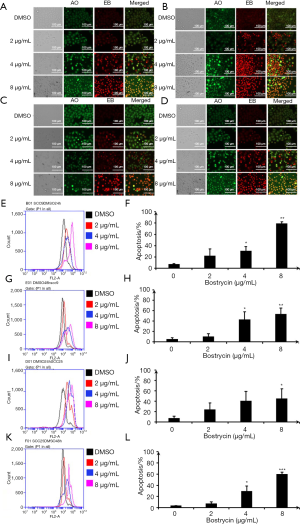
As shown in Figure 4, SCC9 and SCC25 cells were treated with bostrycin for 24 and 48 hours, respectively. The apoptotic cells were detected by AO/EB staining. When treated with 2 µg/mL Bostrycin, a small amount of red fluorescence appeared. When treated with 4 µg/mL, the red fluorescence increased, and when treated with 8 µg/mL, a large amount of red fluorescence occurred (Figure 4A,B,C,D). These results showed that the apoptotic rate increased with the increasing of the concentration of Bostrycin. The results of AO/EB staining analyzed by flow cytometry showed that the red fluorescence was shifted to the right, indicating that the dead cells were increased with the increasing of the concentration of Bostrycin. When treated with bostrycin for 24 hours, the apoptotic rate of SCC9 cells was increased by 14.93% to 71.8%. The apoptotic rate of SCC9 cells was increased by 5.30% to 48.23%, Figure 4E,F). When treated for 48 h, the apoptotic rate of SCC9 cells was increased by 23.4% at 4 µg/mL and 48.23% at 8 µg/mL, respectively (Figure 4G,H). The apoptotic rate of SCC9 cells was significantly higher than that of the control group. The apoptotic rate of SCC25 cells treated with bostrycin at 2 µg/mL for 24 hours was 16.77%. And that of SCC25 was 33.07% at 4 µg/mL and 37.57% at 8 µg/mL, respectively (Figure 4I,J). When treated with 4 µg/mL bostrycin for 48 hours, the apoptotic rate of SCC25 cells was increased by 26.03% at 4 µg/mL and 56.17% at 8 µg/mL, respectively (Figure 4K,L). When treated with Bostrycin, the apoptotic rate of SCC25 cells was significantly higher than that of the control group.
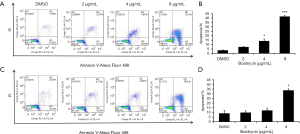
Further detection of apoptosis was executed by flow cytometry with double staining of Annexin V/PI, after SCC9 and SC25 were treated with bostrycin for 24 hours (Figure 5). The results showed that apoptosis of SCC9 cells were increased by 3.7% at 2 µg/mL, 10.5% at 4 µg/mL, 38.2% at 8 µg/mL (Figure 5A,B), and that of SCC25 were raised 0.8% at 2 µg/mL, 3.1% at 4 µg/mL and 24.6% at 8 µg/mL (Figure 5C,D). Both early and later apoptosis rates of SCC9 and SCC25 cells were significantly induced by Bostrycin.
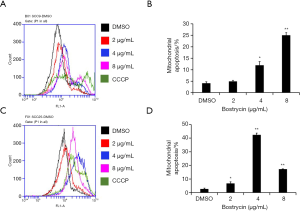
Mitochondrial membrane potential changes of tongue squamous cell carcinoma cells were induced by Bostrycin
JC-1 assay was used to detect the effect of bostrycin on mitochondrial membrane potential of tongue squamous cell carcinoma cells. The results showed that the mitochondrial apoptotic rates of SCC9 cells were increased by 0.75%, 7.8% and 20.9%, when the concentration of bostrycin were 2, 4 and 8 µg/mL, respectively (Figure 6A,B). The mitochondrial apoptotic rate of SCC25 cells was increased by 39.8% when the concentration was 4 µg/mL (Figure 6C,D). The results showed that mitochondrial apoptosis of SCC25 cells was more sensitive to Bostrycin. The apoptotic rate of SCC25 cells treated with 4 µg/mL was much higher than that of SCC9 cells. However, when SCC25 cells were treated with 8 µg/mL, most of the cells died, which led to the low detection of apoptotic rate.
Bostrycin induced apoptosis of tongue squamous cell carcinoma cells through mitochondrial signaling pathway
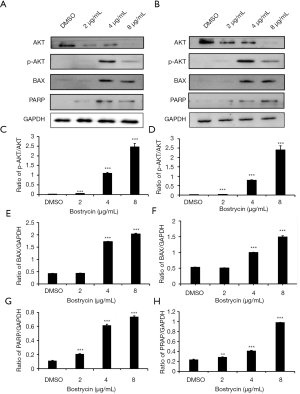
Western blot assay was used to detect the expression or activation of related proteins in apoptotic signaling pathway (Figure 7). The results showed that the phosphorylation of Akt increased gradually with the raising of bostrycin concentration, suggesting that bostrycin may induce cell apoptosis through Akt. Meanwhile, the expressing of Bax and PRAP were elevated under the same condition, which mean the apoptosis induced by bostrycin should been through mitochondrial apoptotic pathways.
Discussion
The colony of endophytic fungi HCH285 is round, white villus and fluffy at the beginning of growth, and the red metabolite pigments can be seen clearly on the back of the colony at later stage. Monomers compounds were separated by semi-preparative liquid phase from the pigments extracted by varied chemical reagents, and finally analyzed as bostrycin by NMR. bostrycin is a natural organic anticancer derivative with anthraquinone structure, which is slightly soluble in sterile water and DMSO. In Toxicity analysis of bostrycin and HCH285 pigments, C. elegans was introduced as the biological material, and food grade Monascus was used as the control to study the effects of different nematode indicators. The results showed that the pigments produced by HCH285 had no significant inhibitory effect on the acute and chronic toxicity, feeding and exercise ability, life history status, egg hatching rate of C. elegans, as the same as effect with food grade Monascus. When none of the indicators for nematodes had toxic effect, the safe dosage of HCH285 was 500 µg/mL, and the safe dosage of bostrycin was 10 µg/mL (17).
There were researches showed that IC50 of bostrycin was 4.08 µM on normal epithelial cells, which was 2-7 times as effective as that on cancer cells. This indicated that although bostrycin had a weak toxic effect on normal cells, it had the potential to be modified as an anticancer drug in the future. Yongcheng Lin and Zhigang She’s research groups have proved that bostrycin could inhibit the growth of breast cancer cells and induce apoptosis through Akt/FOXO pathway (6). bostrycin also suppressed the proliferation of human lung cancer A549 cells by down-regulating P13K/Akt (18). The derivatives of bostrycin refrained the growth of hepatocellular carcinoma cell Hep G2, breast cancer cell MDA-MB-435 and colon cancer cell HCT-116 (19). A large number of data have demonstrated that bostrycin has broad-spectrum anticancer activity including lung cancer cells A549, breast cancer MCF-7 and MDA-MB435A cells, colon cancer HCF-116 cells, liver cancer Hep G2 cells, gastric cancer MCG803 cells and so on. The half-inhibition concentration ranges from 2.18 to 7.71 mM, and that of on epithelial MCF-10A cells is 14.08 mM. Beside Yongcheng Lin and Zhigang She’s Research groups on Bostrycin, Chuangqi Chen found that bostrycin could significantly inhibit the growth of gastric cancer cell MCG-803, and speculated that it might induce cell apoptosis through mitochondrial apoptotic pathway (20).
In this study, bostrycin was used to exam its inhibitory effect on tongue squamous cell carcinoma cells SCC9 and SCC25, and to explore its mechanism. These results showed that bostrycin could effectively inhibit the proliferation of tongue squamous cell carcinoma cells. MTT assay showed that the IC50 of bostrycin was 5.37 µg/mL on SCC9 cells and 3.50 µg/mL on SCC25 cells. It is concluded that bostrycin behaved good anticancer activity on tongue squamous cell carcinoma cells in vitro, especially inhibition effect on SCC25 cell proliferation. Cell cycle experiments showed that bostrycin inhibited the proliferation of tongue squamous cell carcinoma cells by inducing G2/M phase arresting. Cycle detection also exhibited that with the increasing of bostrycin concentration, the small apoptotic peak of sub-G1 phase increased significantly, which suggested that bostrycin might induce apoptosis of tongue squamous cell carcinoma cells. AO/EB staining and Annexin V/PI double staining experiments displayed that bostrycin cells could promote apoptosis of tongue squamous carcinoma cells. JC-1 assay showed that bostrycin could lead the changes of mitochondrial membrane potential of tongue squamous cell carcinoma cells. Western blot assay showed that bostrycin could significantly increase the phosphorylation of AKT, which may relate to the apoptosis of tongue squamous carcinoma cells. The results showed that the expression of BAX and PRAP were increased, which indicated that bostrycin may affect apoptosis through mitochondrial apoptosis pathway.
Conclusions
In conclusion, bostrycin has good inhibitory effect on tongue squamous cell carcinoma in vitro. bostrycin induced apoptosis through mitochondrial apoptotic pathway, which were demonstrated both by the changes of mitochondrial membrane potential and the expression of BAX. BAX-dependent mitochondrial permeabilization during apoptosis is controlled by multiple factors, including the phosphorylation by the protein kinase AKT. However, the particular mechanism of mitochondrial apoptosis induced by bostrycin in tongue squamous carcinoma cells remains to be further studied.
Acknowledgments
Funding: This work was supported by grants from
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at: http://dx.doi.org/10.21037/tcr-19-2076). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Huang C, Shen FF, Li G, et al. Effects of exenatide on the cell proliferation, invasion and apoptosis of human tongue squamous cell carcinoma SCC-25. Tianjin Med J 2015;43:461-4.
- Wu KL, Li SY, Zhang KP. Research progress in biological property of Streptococcus mutans glucan binding protein B. Int J Stom 2015;42:119-22.
- Zhaoqiang Z, Qingbin Z, Lei C, et al. Infant ectopic cervical thymus one case report: diagnostic and management difficulties. J Craniomaxillofac Surg 2012;40:701-5. [Crossref] [PubMed]
- Ghosh S, Koblinski J, Johnson J, et al. Urinary-type plasminogen activator receptor/alpha 3 beta 1 integrin signaling, altered gene expression, and oral tumor progression. Mol. Cancer Res 2010;8:145-58. [Crossref] [PubMed]
- Noda T, Take T, Otani M, et al. Structure of Bostrycin. Tetrahedron Lett 1968;9:6087-90. [Crossref] [PubMed]
- Chen H, Zhong L, Long Y, et al. Studies on the Synthesis of Derivatives of Marine-Derived bostrycin and Their Structure-Activity Relationship against Tumor Cells. Mar Drugs 2012;10:932-52. [Crossref] [PubMed]
- Yin H, Que R, Liu C, et al. Survivin-targeted drug screening platform identifies a matrine derivative WM-127 as a potential therapeutics against hepatocellular carcinoma. Cancer Lett 2018;425:54-64. [Crossref] [PubMed]
- Sun FD, Wang PC, Shang J, et al. Ibrutinib presents antitumor activity in skin cancer and induces autophagy. Eur Rev Med Pharmacol Sci 2018;22:561-6. [PubMed]
- Chen Z, Zuo X, Pu L, et al. circLARP4 induces cellular senescence through regulating miR-761/RUNX3/p53/p21 signaling in hepatocellular carcinoma. Cancer Sci 2019;110:568-81. [Crossref] [PubMed]
- Fan D, Lin X, Zhang F, et al. MicroRNA 26b promotes colorectal cancer metastasis by downregulating phosphatase and tensin homolog and wingless-type MMTV integration site family member 5A. Cancer Sci 2018;109:354-62. [Crossref] [PubMed]
- Liu J, Bian T, Feng J, et al. miR-335 inhibited cell proliferation of lung cancer cells by target Tra2β. Cancer Sci 2018;109:289-96. [Crossref] [PubMed]
- Zhang Y, Huang L, Shi H, et al. Ursolic acid enhances the therapeutic effects of oxaliplatin in colorectal cancer by inhibition of drug resistance. Cancer Sci 2018;109:94-102. [Crossref] [PubMed]
- Tokala R, Bale S, Pavan Janrao I, et al. Synthesis of 1,2,4-triazole-linked urea/thiourea conjugates as cytotoxic and apoptosis inducing agents. Bioorg Med Chem Lett 2018;28:1919-24. [Crossref] [PubMed]
- Ma ZJ, Lu L, Yang JJ, et al. Lariciresinol induces apoptosis in HepG2 cells via mitochondrial-mediated apoptosis pathway. Eur J Pharmacol 2018;821:1-10. [Crossref] [PubMed]
- Shilnikova K, Piao MJ, Kang KA, et al. Shikonin induces mitochondria-mediated apoptosis and attenuates epithelial-mesenchymal transition in cisplatin-resistant human ovarian cancer cells. Oncol Lett 2018;15:5417-24. [PubMed]
- Hou H, Ge C, Sun H, et al. Tunicamycin inhibits cell proliferation and migration in hepatocellular carcinoma through suppression of CD44s and the ERK1/2 pathway. Cancer Sci 2018;109:1088-100. [Crossref] [PubMed]
- Suwannarach N, Kumla J, Nishizaki Y, et al. Optimization and characterization of red pigment production from an endophytic fungus, Nigrospora aurantiaca CMU-ZY2045, and its potential source of natural dye for use in textile dyeing. Appl. Microbiol. Biotechnol 2019;103:6973-87. [Crossref] [PubMed]
- Chen WS, Hou JN, Guo YB, et al. bostrycin inhibits proliferation of human lung carcinoma A549 cells via downregulation of the PI3K/Akt pathway. J Exp Clin Cancer Res 2011;30:17. [Crossref] [PubMed]
- Onozawa T, Kitajima M, Kogure N, et al. Asymmetric Total Synthesis and Evaluation of Antitumor Activity of Ophiorrhisine A and Its Derivatives. J Org Chem 2018;83:15312-22. [Crossref] [PubMed]
- Chen CQ, Fang LK, Liu JW, et al. Effects of marine fungal metabolites 1386A from the South China Sea on proliferation, apoptosis and mitochondrial membrane potential in gastric cancer cell line MCG-803. Chin J Pathophys 2010;26:1908-12.


