
Biomarkers and the role of mast cells as facilitators of inflammation and fibrosis in chronic kidney disease
Introduction
The kidney is integral to maintaining homeostasis, with one of its main roles being the removal of waste substances from the blood (1). Chronic kidney disease (CKD) is a clinical syndrome with many adverse sequelae. It is stratified by estimated glomerular filtration rate (eGFR) and encompasses all causes of kidney dysfunction resulting in reduced eGFR, with or without other structural or functional abnormalities, present for at least 3 months (2). Globally, CKD was the 18th most prevalent cause of death, and had an annual incidence rate of 16.3 per 100,000 people from 1990–2012 in the developed world (3). In Australia, 1 in 10 people are affected. The total cost attributed to CKD in 2012 was estimated at $4.1 billion and this was expected to rise annually (4,5). Despite the impact on human health and society, there are no successful targeted treatments to slow development of CKD and circumvent CKD progression to end stage kidney disease (ESKD). There are also few successful biomarkers for indicating early development and progression of CKD.
Regardless of aetiology, inflammation and fibrosis are common manifestations of CKD. Inflammation is characterised by excessive innate and adaptive immune responses with infiltration of inflammatory cells and the release of cytokines Fibrosis is characterised by increasing presence of extracellular matrix (ECM) proteins that gradually replace normal tissue architecture, and prevent normal functioning of specialised kidney cells such as tubular epithelial cells, podocytes and mesangial cells in the glomeruli, and vascular endothelial cells (6). Normally, inflammation is a protective response to potentially harmful endogenous or exogenous causes of injury, aiming to eliminate cellular threats and promoting tissue repair through fibrosis. Protracted inflammation, however, is damaging and the associated fibrogenesis can result in organ failure if not corrected (7). Unfortunately, the underlying pathophysiological mechanisms of protracted inflammation and fibrosis in CKD, including the role of the immune system, are poorly understood.
This mini review will examine the role of mast cells (MCs), an inflammatory cell that has received little attention in the context of CKD, as a facilitator of kidney inflammation and fibrosis through its degranulation or de novo synthesis of bio-active compounds within the kidney. It will propose a mechanistic structure for MCs in progressive CKD where it can be targeted therapeutically or utilised to slow or circumvent CKD progression. Finally, it will consider what clinical biomarkers of MC activity within kidney disease are available as possible avenues of novel prognostic and diagnostic tools.
MCs
MCs are derived from haematopoietic stem cells within the bone marrow and are characterised by the presence in their cytoplasm of electron-dense granules filled with many preformed mediator compounds. They enter the circulation as progenitors rather than end-stage cells like other myeloid-derived cells (8). MC progenitors migrate through vessels to peripheral tissue, where they reside close to blood vessels, the epithelium and nerves in connective tissue. There they differentiate into different MC subtypes based on local growth factors (9). This distribution and structural interrelationship with tissue-specific cells allows activated MCs to modulate innate immune and adaptive effector responses via the degranulation or de novo synthesis of bio-active compounds (10). The preformed compounds of the granules, which can be heterogeneous in composition, can be grouped into lysosomal enzymes, biogenic amines, cytokines and growth factors, proteoglycans and proteases (11). Bio-active compounds synthesised by MCs include lipid mediators, cytokines and chemokines (12). This range of compounds allows MCs to elicit a variety of immunological responses.
Kidney disease and MCs
MCs are beneficially involved during wound healing. This beneficial role can be negated in some instances, for example, during chronic tissue injury, activated MCs can accumulate and trigger a pathological response (13). In a subtotal nephrectomy rat model, MCs infiltrated the tubulointerstitium, particularly areas of tubular dilatation and interstitial fibrosis, but not within the glomerular tuft. In contrast, in sham-operated rats, MCs were only occasionally observed (14). This distribution pattern was replicated in a puromycin aminonucleoside nephrosis model of glomerular disease in mice (15).
A similar pattern is observed in humans with chronic rejection of kidney allografts and various native kidney diseases. Compared to normal kidney tissue, MC infiltration in diseased tissue is increased and preferentially localises within the interstitium and, rarely, within the glomeruli (16-24). This infiltration positively correlates with the clinical kidney function markers of blood urea nitrogen, serum creatinine and urinary protein at the time of tissue collection. For example, MC infiltration was correlated with an increase in serum creatinine between tissue collection and follow up in IgA nephropathy (19,21). Interstitial fibrosis, a common manifestation of kidney disease, was positively correlated with the degree of MC infiltration (17,20-22,25). Heightened levels of MCs were also associated with worse clinical outcomes in patients with kidney disease, while those with stable or improving renal function had reduced MC infiltration (19,20). In cases of chronic kidney transplant rejection, MC infiltration increased with the grade of rejection, determined by degree of interstitial fibrosis, oedema, and haemorrhage, and was increased over healthy controls (26). Allograft biopsies also indicated that fibrotic scarring, impaired graft survival and impaired functional recovery were associated with increased expression of MC transcripts (27).
The heterogeneous composition of MC granules and de novo synthesis of bio-active compounds means MCs are capable of inducing a range of immunological responses as an effector population of the immune system. Further, MCs are capable of rapidly responding to tissue because preformed mediator compounds are stored within granules in their active forms (28). Wernersson and Pejler have described in detail the local effects of MC degranulation (11). This includes stimulation of afferent nerve cells, smooth muscle contraction, vasodilatation and vascular permeability, inflammation and fibrosis through released chemokines and cytokines, protein degradation by proteases, and distant effects via circulating granule remnants. As the kidney is a highly vascularised organ, MC-induced vasodilation and increased vascular permeability will, potentially, allow for greater recruitment and infiltration of immunological cells into kidney tissue. Moreover, the type and level of chemokines, cytokines and proteases (11,12) will have pro-inflammatory and pro-fibrotic actions within the kidney in their own right. Those of primary interest are tumour necrosis factor-α (TNF-α), monocyte chemoattractant protein-1 (MCP-1), interleukin-6 (IL-6), transforming growth factor-β (TGF-β) and tryptase.
TNF-α is increased in both CKD and ESKD patients when compared to healthy controls (29-31). These elevated levels have also been linked to worse outcomes in CKD patients. A multivariate model showed CKD patients in the highest TNF-α tertile were associated with increased risk of CKD progression (32), while elevated plasma TNF-α concentrations were associated with a greater risk of kidney functional decline (33). Increased TNF-α was also associated with lower eGFR and serum albumin and higher albuminuria (32,33). Baseline measurements of the chemokine MCP-1 are capable of predicting more rapid eGFR decline in CKD patients (30) and increased MCP-1 expression, as a ratio of creatinine, is associated strongly with sustained functional decline in progressive CKD patients (34-36). Kidney biopsies taken from CKD patients indicate increased IL-6 expression in both the glomeruli and tubules when compared to healthy controls (37), while plasma IL-6 levels are elevated in ESKD patients compared to healthy controls (31,38,39). Increases in IL-6 levels have been associated with eGFR decline over time (33) and are predictive of poor outcomes and survival (40,41). TGF-β is an established driver of glomerular and tubulointerstitial fibrosis within CKD. In a number of kidney cell types, TGF-β can induce production of ECM proteins (42-45), in addition to inducing endothelial-to-mesenchymal and epithelial-to-mesenchymal transition (46), two potential sources of myofibroblasts and ECM in kidney disease. Proliferation and hypertrophy of kidney cells, associated with CKD, can be induced by TGF-β (42-45,47), along with inducing apoptosis of podocytes (42,48,49), epithelial and endothelial cells (43,50).
Protease activated receptor 2 (PAR-2) as a potential target of MCs in kidney disease
PAR-2, a G-protein coupled receptor (GPCR), has recently been shown to induce pro-inflammatory and pro-fibrotic responses within the kidney and other organs. PAR-2 is, potentially, involved in the pathological mechanisms of CKD through its activation by secreted MC products and coagulation factors upregulated during CKD (Figure 1). This can result in robust pro-inflammatory and pro-fibrotic responses from cells of the kidney, mediated by PAR-2. PAR-2 has been suggested to regulate and augment cellular responses through its ability to undergo homo- or hetero-dimerisation as well as via the transactivation of the epidermal growth factor (EGF) and TGF-β receptors (51), both of which have pro-fibrotic effects within the kidney. This positions MCs and PAR-2 activation as mechanisms of inflammation and fibrosis associated with CKD.
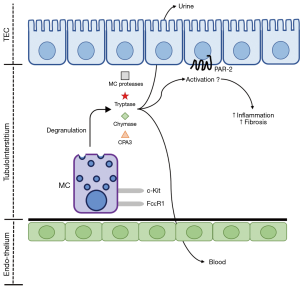
In the human kidney, PAR-2 is expressed broadly in podocytes, tubular epithelial, mesangial, collecting duct, inflammatory and fibroblastic cells and appears to be involved primarily in inflammation and fibrosis when expressed within the diseased kidney (52-57). Biopsies from IgA nephropathy patients show PAR-2 expression is increased, particularly in patients with moderate and severe lesions, when compared to healthy kidneys (58). Despite the widespread expression of PAR-2 in the kidney, its enhanced expression during disease is preferentially located in proximal tubular epithelial cells in animal models of kidney fibrosis, such as the unilateral ureteral obstruction (UUO) model (51,57) and in IgA nephropathy (58).
While PAR-2 is likely being activated during CKD as a result of increased MC infiltration, it is important to consider what impact PAR-2 signalling might have on inflammation and fibrosis associated with CKD. PAR-2 may transactivate the receptors of one of the key regulators of fibrosis in the kidney, TGF-β (59,60). TGF-β has a pro-fibrotic action in the kidney through direct effects in activating chronic inflammatory cells such as fibroblasts and macrophages, and indirect effects in epithelial-to-mesenchymal transition, and also in causing apoptosis in the endothelium and podocytes (60). Apart from fibrosis, many physiological responses are also regulated by TGF-β, including immune responses, tissue repair, and apoptosis (61). Isoforms of the TGF-β family include TGF-β1, -β2, and -β3, and the TGF-β receptors include TGFR1 and TGFR2. TGF-β exists in latent and activated forms; in the latent form it complexes with a latency-associated protein (LAP) and the latent TGF-β-binding protein (LTBP); and it is activated through cleavage by a number of proteases (62). For example, ECM cleavage by matrix metalloproteinases (MMP) Facilitates the liberation of preformed cytokines (including TGF-β) anchored to the plasma membrane (63). Notably, treating human kidney proximal tubular epithelial cells with the synthetic PAR-2 peptide SLIGKV-NH2 has been shown to induce expression of MMP-1 (56). MMPs have also previously been reported to transactivate the EGF receptor (62,64,65), and this transactivation has been linked to PAR-2. For example, the treatment of human tubular epithelial cells with the synthetic PAR-2 activator 2f-LIGRLO-NH2 plus TGF-β1 has been shown to synergistically increase EGF receptor activation, as demonstrated by increased ERK1/2 phosphorylation. This transactivation was mediated partially via MMPs, with the pan-MMP inhibitor marimastat partially inhibiting this increased ERK1/2 phosphorylation (66).
Progression in CKD—a need for new biomarkers
Clinicians approach CKD with the therapeutic goal of preventing or slowing progression of the disease. However, not all patients progress at the same rate or at all. In the RENAAL study of patients with type 2 diabetes mellitus and nephropathy, only 22.5% of participants developed ESKD in the following years (67). In the Go-Darts cohort, only 12.5% of stage 3 CKD patients lost >40% of their baseline eGFR (68). Further, in an African American population with CKD related to hypertension, 3.3% of patients showed improved kidney function over a period of 140 months (69). The Chronic Kidney Disease Queensland (CKD.QLD) Registry has confirmed the varying progression rates in CKD patients. Over a 2-year period, 29% had continual decline, 24% declined and then improved, 4% declined and then stabilised, 10% continually improved, 23% improved and then declined, 1% improved and then stabilised, 1% had continued stabilisation, 4% were stable and then declined, and 3% were stable and then improved (70). Thus, CKD progression is not inevitable and an undifferentiated approach to treatment and research will be wasted or inconclusive if not targeted to progressive CKD patients. Currently, there is a lack of validated prognostic CKD biomarkers. The current diagnostic markers (outlined by the Kidney Disease Improving Global Outcomes or KDIGO guidelines) are inadequate in terms of prognostic value for clinical and research goals. Thus, new prognostics and diagnostic biomarkers of CKD are needed.
Identification of MCs in kidney disease has relied primarily on histochemical approaches (for example, toluidine blue or Alcian blue with nuclear fast red stains) or immunohistochemical detection of MC-specific proteases, such as tryptase or chymase, in combination with MC-specific biomarkers c-Kit and FcεR1. These methods would require core biopsies of the kidney, which are neither common nor useful for early diagnosis or prognostic assessment because of the invasive nature of the procedure. Thus, a biomarker measurable in the blood or urine of CKD patients is required. While many of the compounds secreted by MCs can be detected in blood or urine, many of these are non-specific biomarkers. Tryptase, chymase and carboxypeptidase A3 (CPA3), however, are MC-specific proteases (11,12). Detection of these above healthy levels in the blood or urine of a CKD patient would be a non-invasive biomarker of MC activity.
Tryptase, chymase and CPA3 have previously been examined as biomarkers of MC activity in non-kidney related diseases. In the context of kidney disease, these biomarkers have received little attention as potential clinical tools (Figure 1), with the studies that have been conducted narrow in scope. Normal serum levels of tryptase have been established, with total tryptase confirmed at <15 ng/mL, while mature tryptase is confirmed at <1 ng/mL. Total serum tryptase was increased in patients with anaphylaxis and mastocytosis, a condition characterised by the abnormal accumulation of MCs in in the skin and/or internal organs (71). In CKD patients, serum tryptase levels were higher in men compared to women and the concentration was increased in stages 4 and 5 CKD and haemodialysis patients compared to stages 1 and 2 CKD. This increase was negatively correlated with albumin, creatinine clearance, eGFR and urine creatinine (72). A post mortem analysis of anaphylaxis-induced death showed elevated chymase levels in serum samples, in addition to being stable across repeated freeze-thaw cycles and incubation at an elevated temperature (73). Further, in a range of paediatric mastocytosis patients, serum chymase was elevated over healthy controls (74). In CKD patients, a remarkable increase in plasma chymase concentration, compared to healthy controls, has been observed (75). Serum CPA3 was increased in paediatric allergic diseases compared to children without allergic disease (76). In adults with suspected anaphylaxis and systemic mastocytosis, serum CPA3 was also increased over healthy controls (77). In addition to being observable in serum, saliva CPA3 concentration was increased in individuals who had suffered a previous episode of anaphylaxis compared to others without this history (78).
While these MC-specific proteases can be easily detected in blood, consideration as to whether these are cleared by the kidney and excreted in the urine must be made. In urine that had been concentrated 10-fold, mature tryptase was undetectable, while total tryptase was detected at very low concentrations (<0.2 ng/mL) for both healthy controls and individuals with idiopathic anaphylaxis and systemic mastocytosis. Despite being lowly expressed in the urine, total tryptase was readily detectable in the serum of these individuals (79). Conversely, a study examining urinary tryptase levels showed increased concentrations in interstitial cystitis patients compared with healthy controls (80). A difference between these two studies was that the former used spot urine collections, while the later used 24-hour urine collection. CPA3 is a member of the carboxypeptidase family. A study showed carboxypeptidase activity, via a colorimetric assay where the substrates Bz-Gly-Lys or Bz-Gly-Arg are hydrolysed to hippuric acid, was present in urine. Compared to healthy controls, carboxypeptidase activity was increased in nephritic glomerulonephritis; however, it was reduced in patients with chronic renal failure (81).
Conclusions
Globally CKD is a major health and economic burden that currently lacks successful targeted therapies to slow development and circumvent progression to ESKD. Additionally, there is a lack of biomarkers for use in early diagnosis and prognosis of CKD. Regardless of aetiology, a common manifestation of CKD is inflammation and fibrosis within the kidney. MCs are myeloid-derived cells that modulate innate immune and adaptive effector responses through a range of preformed compounds stored within granules or synthesised bio-active compounds. This population of cells has received little attention in the context of CKD, despite being closely linked to a variety of kidney diseases, kidney dysfunction, and adverse clinical outcomes of CKD patients. Here we have presented several mechanistic pathways in which MCs are involved in the development and progression of kidney disease, in addition to suggesting several clinically-useful biomarkers of MC activity. Further work is required to elucidate the pathophysiological role of MCs in CKD and validate clinical biomarkers.
Acknowledgments
The authors would like to acknowledge the staff of the Kidney Disease Research Collaborative at The University of Queensland and the staff of the National Health and Medical Research Council Chronic Kidney Disease Centre of Research Excellence for their help in preparing this manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Skorecki K, Chertow GM, Marsden PA, et al. Brenner and Rector's The Kidney. 10 ed. Elsevier Health Sciences, 2015.
- Kidney Health Australia. Chronic Kidney Disease (CKD) Management in General Practice: Guidance and clinical tips to help identify, manage and refer patients with CKD in your practice. 3rd ed. Melbourne, Australia: Kidney Health Australia, 2015.
- Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2095-128. [Crossref] [PubMed]
- Australian Bureau of Statistics. Australian Health Survey: Biomedical Results for Chronic Disease, 2011-12. Australian Government. 2013.
- Wyld ML, Lee CM, Zhuo X, et al. Cost to government and society of chronic kidney disease stage 1-5: a national cohort study. Intern Med J 2015;45:741-7. [Crossref] [PubMed]
- Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med 2012;18:1028-40. [Crossref] [PubMed]
- Meng XM, Nikolic-Paterson DJ, Lan HY. Inflammatory processes in renal fibrosis. Nat Rev Nephrol 2014;10:493-503. [Crossref] [PubMed]
- Murphy KM. Janeway's Immunobiology. 8th ed. New York, United States of America: Garland Science, 2012.
- Gurish MF, Austen KF. Developmental origin and functional specialization of mast cell subsets. Immunity 2012;37:25-33. [Crossref] [PubMed]
- Holdsworth SR, Summers SA. Role of mast cells in progressive renal diseases. J Am Soc Nephrol 2008;19:2254-61. [Crossref] [PubMed]
- Wernersson S, Pejler G. Mast cell secretory granules: armed for battle. Nat Rev Immunol 2014;14:478-94. [Crossref] [PubMed]
- Moon TC, Befus AD, Kulka M. Mast cell mediators: their differential release and the secretory pathways involved. Front Immunol 2014;5:569. [Crossref] [PubMed]
- Douaiher J, Succar J, Lancerotto L, et al. Development of mast cells and importance of their tryptase and chymase serine proteases in inflammation and wound healing. Adv Immunol 2014;122:211-52. [Crossref] [PubMed]
- Jones SE, Kelly DJ, Cox AJ, et al. Mast cell infiltration and chemokine expression in progressive renal disease. Kidney Int 2003;64:906-13. [Crossref] [PubMed]
- Miyazawa S, Hotta O, Doi N, et al. Role of mast cells in the development of renal fibrosis: use of mast cell-deficient rats. Kidney Int 2004;65:2228-37. [Crossref] [PubMed]
- Ehara T, Shigematsu H. Contribution of mast cells to the tubulointerstitial lesions in IgA nephritis. Kidney Int 1998;54:1675-83. [Crossref] [PubMed]
- Kondo S, Kagami S, Kido H, et al. Role of mast cell tryptase in renal interstitial fibrosis. J Am Soc Nephrol 2001;12:1668-76. [PubMed]
- McPherson EA, Luo Z, Brown RA, et al. Chymase-like angiotensin II-generating activity in end-stage human autosomal dominant polycystic kidney disease. J Am Soc Nephrol 2004;15:493-500. [Crossref] [PubMed]
- Silva GE, Costa RS, Ravinal RC, et al. Mast cells, TGF-beta1 and alpha-SMA expression in IgA nephropathy. Dis Markers 2008;24:181-90. [Crossref] [PubMed]
- Hiromura K, Kurosawa M, Yano S, et al. Tubulointerstitial mast cell infiltration in glomerulonephritis. Am J Kidney Dis 1998;32:593-9. [Crossref] [PubMed]
- Kurusu A, Suzuki Y, Horikoshi S, et al. Relationship between mast cells in the tubulointerstitium and prognosis of patients with IgA nephropathy. Nephron 2001;89:391-7. [Crossref] [PubMed]
- Pardo J, Diaz L, Errasti P, et al. Mast cells in chronic rejection of human renal allografts. Virchows Arch 2000;437:167-72. [Crossref] [PubMed]
- Rüger BM, Hasan Q, Greenhill NS, et al. Mast cells and type VIII collagen in human diabetic nephropathy. Diabetologia 1996;39:1215-22. [Crossref] [PubMed]
- Tóth T, Toth-Jakatics R, Jimi S, et al. Mast cells in rapidly progressive glomerulonephritis. J Am Soc Nephrol 1999;10:1498-505. [PubMed]
- Yamada M, Ueda M, Naruko T, et al. Mast cell chymase expression and mast cell phenotypes in human rejected kidneys. Kidney Int 2001;59:1374-81. [Crossref] [PubMed]
- Lajoie G, Nadasdy T, Laszik Z, et al. Mast cells in acute cellular rejection of human renal allografts. Mod Pathol 1996;9:1118-25. [PubMed]
- Mengel M, Reeve J, Bunnag S, et al. Molecular correlates of scarring in kidney transplants: the emergence of mast cell transcripts. Am J Transplant 2009;9:169-78. [Crossref] [PubMed]
- Palm NW, Rosenstein RK, Medzhitov R. Allergic host defences. Nature 2012;484:465-72. [Crossref] [PubMed]
- Lucisano S, Arena A, Stassi G, et al. Role of Paricalcitol in Modulating the Immune Response in Patients with Renal Disease. Int J Endocrinol 2015;2015:765364. [Crossref] [PubMed]
- Verhave JC, Bouchard J, Goupil R, et al. Clinical value of inflammatory urinary biomarkers in overt diabetic nephropathy: a prospective study. Diabetes Res Clin Pract 2013;101:333-40. [Crossref] [PubMed]
- Khozeymeh F, Mortazavi M, Khalighinejad N, et al. Salivary levels of interleukin-6 and tumor necrosis factor-alpha in patients undergoing hemodialysis. Dent Res J (Isfahan) 2016;13:69-73. [Crossref] [PubMed]
- Oh YJ, An JN, Kim CT, et al. Circulating Tumor Necrosis Factor alpha Receptors Predict the Outcomes of Human IgA Nephropathy: A Prospective Cohort Study. PLoS One 2015;10:e0132826. [Crossref] [PubMed]
- Amdur RL, Feldman HI, Gupta J, et al. Inflammation and Progression of CKD: The CRIC Study. Clin J Am Soc Nephrol 2016;11:1546-56. [Crossref] [PubMed]
- Nadkarni GN, Rao V, Ismail-Beigi F, et al. Association of Urinary Biomarkers of Inflammation, Injury, and Fibrosis with Renal Function Decline: The ACCORD Trial. Clin J Am Soc Nephrol 2016;11:1343-52. [Crossref] [PubMed]
- Titan SM, Vieira JM Jr, Dominguez WV, et al. Urinary MCP-1 and RBP: independent predictors of renal outcome in macroalbuminuric diabetic nephropathy. J Diabetes Complications 2012;26:546-53. [Crossref] [PubMed]
- Camilla R, Brachemi S, Pichette V, et al. Urinary monocyte chemotactic protein 1: marker of renal function decline in diabetic and nondiabetic proteinuric renal disease. J Nephrol 2011;24:60-7. [Crossref] [PubMed]
- Zhang W, Wang W, Yu H, et al. Interleukin 6 underlies angiotensin II-induced hypertension and chronic renal damage. Hypertension 2012;59:136-44. [Crossref] [PubMed]
- Babaei M, Dashti N, Lamei N, et al. Evaluation of plasma concentrations of homocysteine, IL-6, TNF-alpha, hs-CRP, and total antioxidant capacity in patients with end-stage renal failure. Acta Med Iran 2014;52:893-8. [PubMed]
- Prakash S, Sarangi AN, Tripathi G, et al. Prediction of susceptible biomarkers for end stage renal disease among North Indians. Nephrology (Carlton) 2016;21:592-600. [Crossref] [PubMed]
- Beberashvili I, Sinuani I, Azar A, et al. IL-6 levels, nutritional status, and mortality in prevalent hemodialysis patients. Clin J Am Soc Nephrol 2011;6:2253-63. [Crossref] [PubMed]
- Pecoits-Filho R, Barany P, Lindholm B, et al. Interleukin-6 is an independent predictor of mortality in patients starting dialysis treatment. Nephrol Dial Transplant 2002;17:1684-8. [Crossref] [PubMed]
- Gruden G, Perin PC, Camussi G. Insight on the pathogenesis of diabetic nephropathy from the study of podocyte and mesangial cell biology. Curr Diabetes Rev 2005;1:27-40. [Crossref] [PubMed]
- López-Hernández FJ, Lopez-Novoa JM. Role of TGF-beta in chronic kidney disease: an integration of tubular, glomerular and vascular effects. Cell Tissue Res 2012;347:141-54. [Crossref] [PubMed]
- Wolf G, Ziyadeh FN. Molecular mechanisms of diabetic renal hypertrophy. Kidney Int 1999;56:393-405. [Crossref] [PubMed]
- Wolf G, Ziyadeh FN. Cellular and molecular mechanisms of proteinuria in diabetic nephropathy. Nephron Physiol 2007;106:26-31. [Crossref] [PubMed]
- Li J, Qu X, Bertram JF. Endothelial-myofibroblast transition contributes to the early development of diabetic renal interstitial fibrosis in streptozotocin-induced diabetic mice. Am J Pathol 2009;175:1380-8. [Crossref] [PubMed]
- Ziyadeh FN, Wolf G. Pathogenesis of the podocytopathy and proteinuria in diabetic glomerulopathy. Curr Diabetes Rev 2008;4:39-45. [Crossref] [PubMed]
- Schiffer M, Bitzer M, Roberts IS, et al. Apoptosis in podocytes induced by TGF-beta and Smad7. J Clin Invest 2001;108:807-16. [Crossref] [PubMed]
- Wolf G, Chen S, Ziyadeh FN. From the periphery of the glomerular capillary wall toward the center of disease: podocyte injury comes of age in diabetic nephropathy. Diabetes 2005;54:1626-34. [Crossref] [PubMed]
- Böttinger EP, Bitzer M. TGF-beta signaling in renal disease. J Am Soc Nephrol 2002;13:2600-10. [Crossref] [PubMed]
- Chung H, Ramachandran R, Hollenberg MD, et al. Proteinase-activated receptor-2 transactivation of epidermal growth factor receptor and transforming growth factor-beta receptor signaling pathways contributes to renal fibrosis. J Biol Chem 2013;288:37319-31. [Crossref] [PubMed]
- Bertog M, Letz B, Kong WY, et al. Basolateral proteinase-activated receptor (PAR-2) induces chloride secretion in M-1 mouse renal cortical collecting duct cells. J Physiol 1999;521:3-17. [Crossref] [PubMed]
- Borensztajn K, Stiekema J, Nijmeijer S, et al. Factor Xa stimulates proinflammatory and profibrotic responses in fibroblasts via protease-activated receptor-2 activation. Am J Pathol 2008;172:309-20. [Crossref] [PubMed]
- D'Andrea MR, Derian CK, Santulli RJ, et al. Differential expression of protease-activated receptors-1 and -2 in stromal fibroblasts of normal, benign, and malignant human tissues. Am J Pathol 2001;158:2031-41. [Crossref] [PubMed]
- Tanaka M, Arai H, Liu N, et al. Role of coagulation factor Xa and protease-activated receptor 2 in human mesangial cell proliferation. Kidney Int 2005;67:2123-33. [Crossref] [PubMed]
- Vesey DA, Suen JY, Seow V, et al. PAR2-induced inflammatory responses in human kidney tubular epithelial cells. Am J Physiol Renal Physiol 2013;304:F737-50. [Crossref] [PubMed]
- Xiong J, Zhu Z, Liu J, et al. Role of protease activated receptor-2 expression in renal interstitial fibrosis model in mice. J Huazhong Univ Sci Technolog Med Sci 2005;25:523-6. [Crossref] [PubMed]
- Grandaliano G, Pontrelli P, Cerullo G, et al. Protease-activated receptor-2 expression in IgA nephropathy: a potential role in the pathogenesis of interstitial fibrosis. J Am Soc Nephrol 2003;14:2072-83. [Crossref] [PubMed]
- Eddy AA. Progression in chronic kidney disease. Adv Chronic Kidney Dis 2005;12:353-65. [Crossref] [PubMed]
- Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-beta: the master regulator of fibrosis. Nat Rev Nephrol 2016;12:325-38. [Crossref] [PubMed]
- Meng XM, Chung AC, Lan HY. Role of the TGF-beta/BMP-7/Smad pathways in renal diseases. Clin Sci (Lond) 2013;124:243-54. [Crossref] [PubMed]
- Ancha HR, Kurella RR, Stewart CA, et al. Histamine stimulation of MMP-1(collagenase-1) secretion and gene expression in gastric epithelial cells: role of EGFR transactivation and the MAP kinase pathway. Int J Biochem Cell Biol 2007;39:2143-52. [Crossref] [PubMed]
- Klein T, Bischoff R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids 2011;41:271-90. [Crossref] [PubMed]
- Carbajal L, Biswas A, Niswander LM, et al. GPCR/EGFR cross talk is conserved in gonadal and adrenal steroidogenesis but is uniquely regulated by matrix metalloproteinases 2 and 9 in the ovary. Mol Endocrinol 2011;25:1055-65. [Crossref] [PubMed]
- Roelle S, Grosse R, Aigner A, et al. Matrix metalloproteinases 2 and 9 mediate epidermal growth factor receptor transactivation by gonadotropin-releasing hormone. J Biol Chem 2003;278:47307-18. [Crossref] [PubMed]
- Chen J, Chen JK, Nagai K, et al. EGFR signaling promotes TGFbeta-dependent renal fibrosis. J Am Soc Nephrol 2012;23:215-24. [Crossref] [PubMed]
- Keane WF, Zhang Z, Lyle PA, et al. Risk scores for predicting outcomes in patients with type 2 diabetes and nephropathy: the RENAAL study. Clin J Am Soc Nephrol 2006;1:761-7. [Crossref] [PubMed]
- Looker HC, Colombo M, Hess S, et al. Biomarkers of rapid chronic kidney disease progression in type 2 diabetes. Kidney Int 2015;88:888-96. [Crossref] [PubMed]
- Hu B, Gadegbeku C, Lipkowitz MS, et al. Kidney function can improve in patients with hypertensive CKD. J Am Soc Nephrol 2012;23:706-13. [Crossref] [PubMed]
- Abeysekera R, Wang Z, Cameron A, et al. Comparison of Different Definitions of Chronic Kidney Disease (Ckd) Progression in Patients in a Metropolitan Public Renal Practice; Queensland, Australia. Nephrology (Carlton) 2016;21 Suppl 2:211.
- Schwartz LB. Diagnostic value of tryptase in anaphylaxis and mastocytosis. Immunol Allergy Clin North Am 2006;26:451-63. [Crossref] [PubMed]
- Sirvent AE, Gonzalez C, Enriquez R, et al. Serum tryptase levels and markers of renal dysfunction in a population with chronic kidney disease. J Nephrol 2010;23:282-90. [PubMed]
- Nishio H, Takai S, Miyazaki M, et al. Usefulness of serum mast cell-specific chymase levels for postmortem diagnosis of anaphylaxis. Int J Legal Med 2005;119:331-4. [Crossref] [PubMed]
- Zhou X, Sanchez-Munoz L, Orfao A, et al. High serum levels of mast cell chymase and carboxypeptidase in mastocytosis. J Allergy Clin Immunol 2013;131:AB56. [Crossref]
- Wasse H, Rivera AA, Huang R, et al. Increased plasma chymase concentration and mast cell chymase expression in venous neointimal lesions of patients with CKD and ESRD. Semin Dial 2011;24:688-93. [Crossref] [PubMed]
- Pan Q, Ding MF, Zhang S, et al. Measurement of plasma mast cell carboxypeptidase and chymase levels in children with allergic diseases. Zhongguo Dang Dai Er Ke Za Zhi 2011;13:814-6. [PubMed]
- Zhou X, Buckley M, Lau L, et al. Mast cell carboxypeptidase as a new clinical marker for anaphylaxis. J Allergy Clin Immunol 2006;117:S85. [Crossref]
- Adabalkareem R, Lau L, Abdelmotelb A, et al. Mast cell tryptase and carboxypeptidase A3 (CPA3) as markers for predicting susceptibility to severe allergic drug reactions. J Allergy Clin Immunol 2017;139:AB39. [Crossref]
- Simon MR, Jan M, Yee J, et al. Tryptase is not cleared by the kidneys into the urine. Int Arch Allergy Immunol 2010;152:28-31. [Crossref] [PubMed]
- Boucher W, el-Mansoury M, Pang X, et al. Elevated mast cell tryptase in the urine of patients with interstitial cystitis. Br J Urol 1995;76:94-100. [Crossref] [PubMed]
- Hamai K, Ikeda R, Sumi H, et al. Carboxypeptidase activity in human urine from healthy subjects and renal disease patients. Clin Chim Acta 1990;188:233-41. [Crossref] [PubMed]


