A Strengths-Weaknesses-Opportunities-Threats (SWOT) analysis on the clinical utility of sperm DNA fragmentation testing in specific male infertility scenarios
Introduction
Infertility affects approximately 15% of couples globally, with male infertility directly or indirectly responsible for about 60% of the cases (1,2). Conditions compromising male fertility potential include varicocele, congenital and genetic abnormalities, endocrine disorders, genitourinary infection, systemic diseases, and exposure to gonadotoxins, which can be identified through a comprehensive male infertility workup (3). A medical history, physical examination, and semen analysis are the minimum standards for the evaluation of men seeking fertility (3). Among these, routine semen analysis is the central laboratory test in male infertility evaluation (4). However, assessment of semen volume, sperm count, sperm motility, and sperm morphology rarely provides robust discriminatory information of the male fertility potential, unless at extremely low levels (5,6). As a result, half of the male infertility cases are deemed as unexplained or idiopathic due to currently existing limitations in diagnostic modalities (7,8).
Sperm DNA plays a critical role in embryo development and therefore influences the chances of establishing a pregnancy and delivering a healthy baby, both natural and assisted (9,10). The conditions that compromise male fertility may cause deterioration in semen parameters and damage sperm DNA (2,11). However, DNA damage is also seen in sperm of men with unexplained and idiopathic infertility (7,12,13). Although no mechanism has been suggested to explain sperm DNA damage conclusively, oxidative stress (OS) has been implicated as an important mediator of infertility in such cases (2,10,12-15). Nonetheless, different intrinsic susceptibility must exist among infertile men with various etiologies, which culminates in the variable effect of OS on sperm DNA (11).
Development of assays to evaluate sperm DNA made it possible to measure the proportion of sperm with damaged chromatin in a given ejaculate. Yet, although often equalized, one has to distinguish between damages directly to the DNA and disturbed chromatin condensation due to improper exchange of the histones by protamines, which in turn renders the DNA vulnerable to damage as the necessary protection provided by protamines is missing. Probes or dyes are used to identify the existence of DNA breaks in specimens examined by fluorescence and optical microscopy or flow cytometry (10,16,17). The term ‘sperm DNA Fragmentation (SDF)’ has been used to name these tests. At present, sperm chromatin structure assay (SCSA), terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL), sperm chromatin dispersion test (SCD), and single gel electrophoresis (Comet) are the most commonly used methods to measure SDF (18).
With increasing evidence on the role of sperm DNA damage in infertility (19) and the possible consequences of damaged sperm chromatin to the health of offspring (9), measurement of SDF in semen became popular as a means of providing additional information to that of routine semen analysis (18). However, a poor understanding of assays’ characteristics and a general belief that SDF is an untreatable condition has hampered the implementation of SDF testing in clinical practice. Among many questions faced by clinicians is the type of test to be used for SDF measurement, the patient populations that might benefit from this test, and the type of remedies to offer the affected patients.
To shed light on these critical issues, a clinical practice guideline (CPG)—the first of its kind—was recently issued concerning the clinical utility of SDF testing in specific clinical scenarios (20). The prime objective of the guideline was to underline the actual indications of SDF testing and to explain the management options available to patients with increased SDF. The authors examined original and review articles concerning the significance of SDF testing and arranged their CPG into two sections. In the first part, they presented the current tests for SDF evaluation, pointing out their core principles as well as the main advantages and shortcomings whereas in the second part they carried out an evidence-based analysis of test utility in clinical scenarios commonly found by urologists and reproductive specialists. Specifically, varicocele, unexplained infertility, recurrent (natural) pregnancy loss, recurrent intrauterine insemination (IUI) failure, in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) failures, and lifestyle risk factors were analyzed. In each clinical scenario, after a detailed discussion of the rationale involved, a clinical recommendation was made by the expert panel (20).
With the publication of the above-mentioned CPG, a group of urologists, andrologists and scientists with expertise in male infertility, as well as gynecologists, reproductive endocrinologists and embryologists were invited to contribute commentaries concerning its utility. These commentaries contained an abundance of information and conflicting views about the clinical utility of SDF testing, which underline the complex nature of SDF. In this review, we examine these commentaries to identify and analyze the strengths and weaknesses of SDF testing as perceived by fertility experts. For this purpose, we applied a Strengths-Weaknesses-Opportunities-Threats (SWOT) analysis, a method that was originally developed for business (21), but has also been adapted to health sciences (22,23). The SWOT analysis focuses on the strengths and weaknesses as a means of identifying threats and opportunities available to circumvent existing gaps that may limit the broad application of a method or system.
Methods
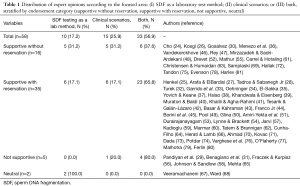
A search of papers published in response to the guideline article by Agarwal et al. (20), entitled “Clinical utility of sperm DNA fragmentation testing: practice recommendations based on clinical scenarios”, was performed within the Translational Andrology and Urology (TAU) website (http://tau.amegroups.com/). The start and end dates for the search were May 2017 and August 2017, respectively. The overall strategy for study identification was based on hand search of ‘ahead of print’ articles related to the guideline mentioned above. Articles’ type included ‘editorial’, ‘commentary’, and ‘letter to the editor’. Additionally, we requested TAU’s editorial office and its senior editor (Dr. Lucine M. Gao) to provide us with full texts of articles to be published by TAU journal that met our inclusion criterion. Fifty-eight relevant articles—from now on termed “commentaries”—were identified and the full (24-43) texts (44-62) were examined (63-81). Each commentary was rated as “supportive without reservation”, “supportive with reservation”, “not supportive” or “neutral” concerning the contents and recommendations provided by the CPG mentioned above. We also noted whether or not the authors specifically discussed the SDF test characteristics and clinical scenarios, and recorded if the concerns raised by them related to SDF as a laboratory test method, clinical scenarios, or both.
Subsequently, we extracted the particulars from each commentary about SDF testing as a lab method and utility of SDF in the clinical scenarios, as applicable. Clinical varicocele with borderline to normal conventional semen parameters, unexplained infertility/recurrent pregnancy loss/IUI failure, IVF/ICSI failure, and borderline abnormal (or normal) conventional semen analysis with lifestyle risk factors were the scenarios contemplated (20). The information was entered into an excel spreadsheet and was categorized further into one of the four areas, namely, “Strengths (positives)”, “Weakness (negatives)”, “Threats (external factors with possible impact)”, and “Opportunities”. Tables outlining the SWOT issues were developed. Then, a critical analysis of each item listed was carried out using both data from existing literature and relevant supporting information provided by a series of ‘reply to author’ published in TAU journal. The latter (82-98) were (99-116) responses (117-133) provided by the guideline’s authors (20) to the commentaries (24-43), editorials (44-62), and letters (63-81).
Results
Descriptive analysis
Fifty-eight fertility experts from six continents and twenty-two countries contributed commentaries. Overall, participants (87.9%; n=51) were supportive of the recommendations provided by the CPG on the utility of SDF testing based on clinical scenarios. Among these, 27.6% (n=16) supported the CPG without reservation and 60.3% (n=35) with reservation, whereas 8.6% (n=5) participants were not supportive or neutral (3.5%; n=2) (Figure 1). The vast majority of participants made specific comments about either the clinical scenarios or SDF assays’ characteristics, or both (Table 1). Among ‘not supportive’ and ‘supportive with reservation’ participants, 75% (n=30/40) and 77.5% (n=31/40) expressed concerns related to technical limitations of SDF testing methods and clinical utility of the test in one or more clinical scenarios discussed in the CPG, respectively (Table 1).

Full table
SWOT analysis
The (24-43) expert (44-62) commentaries (63-81) covered a broad range of issues concerning SDF, which reflect the mixed profile and expertise of participants, as discussed elsewhere (18). As seen in Table 1, authors of commentaries focused either on SDF as a laboratory test method or the utility of such testing in specific clinical scenarios, with most authors covering both areas. Given such heterogeneity, we developed the SWOT analysis in two parts. In the first part, we discuss participants’ concerns regarding SDF as a lab method (Figure 2) and in the second part, we reason their concerns about the clinical utility of such testing in specific clinical scenarios (Figure 3).

Discussion
Part 1: SWOT analysis of SDF as a laboratory test method
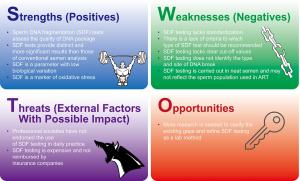
Strengths
SDF tests assess the quality of DNA package
During spermiogenesis, histones are replaced by transition proteins and subsequently with protamines (104,134). Cysteine residues of protamines further undergo intra- and intermolecular disulfide cross-linking resulting in a highly condensed chromatin arranged in a toroid (116). This complex packaging protects the sperm chromatin during its transport from the male to female reproductive tracts and ensures delivery of intact paternal genetic material to the oocyte. A certain amount of histones is, however, retained in human sperm chromatin making it vulnerable to injury (10).
Sperm DNA damage is a complex process involving multiple non-mutually-exclusive causative mechanisms that generate a variety of insults to DNA. Varicocele, infection, and inflammation of the genital tract, cancer, genetic mutations, chromosomal abnormalities, aging, environmental exposure and lifestyle factors are the main conditions associated with sperm DNA damage (2). Furthermore, testicular apoptotic processes during spermatogenesis, aberrations during chromatin remodeling, and OS are among the primary mechanisms postulated to cause nuclear and mitochondrial sperm DNA damage (10,13,49).
SDF testing was originally developed to detect DNA damage in ejaculated sperm (78). Although the term ‘fragmentation’ is widely used to refer to any test that assesses sperm chromatin, not all insults break the DNA into “fragments” (63). In addition to single- or double-stranded DNA breaks, chromatin damage includes DNA nicks, nuclear protein defects, and alteration of chromatin configuration (10). Mostly, the tests measure the proportion of sperm with: (I) real DNA breaks (e.g., TUNEL); (II) combination of actual DNA breaks and potentially denaturable DNA due to the preexistence of single-stranded DNA breaks (SS-DB) or double-stranded DNA breaks (DS-DB) (e.g., SCSA, SCD, and Comet); or (III) poor chromatin packaging (20,104,116). As noted above, not all types of damage are detected by the same test although SDF test results are interrelated to a greater or lesser extent via properties of the DNA (23).
Since the levels of SDF in the neat semen have been associated with infertility, both natural and assisted, it is therefore suggested that SDF testing reflects the overall quality of the DNA package in a given semen specimen, not just the damaged sperm detected in the test result (135).
SDF tests provide results distinct and more significant than those of conventional semen parameters
Overall, clinicians agree that conventional semen analysis has limited value in clinical decision-making (4,9,10,136). Routine laboratory semen analysis is subjected to marked variability due to operational and technical factors (91,117,137-140). Moreover, there is biological variability in semen parameters of same individuals as spermatozoa are mixed with and diluted by fluid secreted from accessory glands, all of which are governed by the state of testicular sperm production, epididymal transit, the activity of accessory glands as well as ejaculatory abstinence (141,142). Importantly, routine semen analysis does not assess essential biological properties of spermatozoa which are needed for fertilization and embryo development. Experience with SDF testing has shown that sperm with high DNA fragmentation can have normal motility and morphology (143), thus helping to explain the limitations of routine semen analysis as to provide robust information to distinguish fertile from infertile men (4,117,136).
In contrast, SDF testing is a more accurate indicator of male fertility status as it looks into the paternal genome. The integrity of sperm DNA is considered to be vital for normal fertilization, embryo development, successful implantation, and pregnancy success in both natural and assisted reproduction [reviewed by Agarwal et al. (19)]. Moreover, in a study evaluating the ability of sperm vitality to predict SDF rates, Samplaski et al. found that over 32% of men gain additional information from SDF testing since vitality test alone would fail to predict SDF in these patients accurately (144). The inclusion of SDF testing to the male infertility armamentarium is advantageous as the test provides valid information that can improve the diagnostic and prognostic value of the routine seminal evaluation (117). Despite the lack of a gold standard test in male infertility, none of the available tests provide diagnostic information equivalent to that offered by SDF during the evaluation of infertile men (19,91).
SDF is a parameter with low biological variation
SDF measurement in consecutive ejaculates is associated with low biological variability. In one study involving 100 men attending an infertility clinic, two consecutive semen analyses were carried out at a median interval of 1.4 months (range: 0.5–6.9) (145). The median coefficient of variation (CV) was significantly lower (P<0.001) in SDF rates by SCSA (9.2%) than that of sperm count (43.0%), progressive motility (28.3%), and normal sperm morphology (28.3%) (145). Others have corroborated these results by showing that the biological variability is significantly less for SCSA results than conventional semen parameters in consecutive samples (146,147).
SDF is a marker of OS
Reactive oxygen species (ROS) are considered the primary cause of SDF (94,122,148,149), thus making SDF testing a candidate surrogate marker of OS. OS is common in the infertile male population and has been associated with many known and unknown infertility conditions, including varicocele, infection, advanced paternal age, heat stress, and lifestyle factors (150,151). Excessive ROS exert a detrimental effect on male fertility via multiple mechanisms including sperm membrane peroxidation, mitochondrial and nuclear DNA damage, and apoptosis. Redox processes using various pathways, including hydroxyl radical, nitric oxide, and activation of sperm caspases and endonucleases, affect not only the sperm membrane but also nuclear and mitochondrial DNA, thus explaining the higher positivity for SDF in live ejaculated sperm of infertile men than fertile counterparts (10,116,152). SDF testing can provide a common pathway to measure the effects of oxidative damage and the success of treatments whether that be varicocele surgery, antioxidants or lifestyle modification (153,154).
Weaknesses
SDF testing lacks standardization
Standardization is necessary for any diagnostic test to be used clinically (4,60,133). Achieving such a characteristic is mainly related to the nature of the test, its complexity, and subjectivity in interpretation. Moreover, accuracy (extent to which the measurement reflects the real value), and precision (reproducibility of test results) are both important for clinicians who rely on the values provided by a laboratory test to direct the workup and counseling of the infertile male (155).
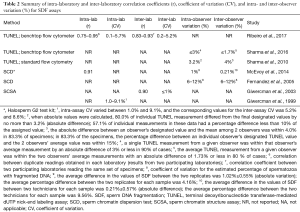
Although some methods to measure SDF suffer from high inter-laboratory variation, many efforts have been made to standardize the SCSA, TUNEL, and the SCD test (110). For instance, inter- and intra-laboratory precision, and inter- and intra-observer CV calculated for SCSA, SCD, and flow-cytometer TUNEL are extremely small (156-162) (Table 2). In a study using the TUNEL assay and a benchtop flow cytometer, semen specimens were evaluated in a blind fashion by two experienced observers, with results showing absolute inter- and intraobserver differences of 1.73% and 6.68% and percent inter- and intraobserver differences of 3% and 9.68% in >80% of cases, respectively (156). With the Halosperm G2 test kit, the intraobserver variability of the absolute average difference in SDF values between replicate tests was 1.02%±0.55% with a mean percent difference of 4.16% (157). In this study, the interobserver variability of SDF values between two technicians showed an absolute difference of 0.21%±0.57% and a mean percent difference of 9.56%. Furthermore, a recent study revealed no significant differences in SDF results between duplicates, with a high correlation between two independent laboratories (r=0.94) when experienced operators used the same semen samples, identical assay kit, protocol and flow cytometer settings to measure SDF by TUNEL (158).
Full table
There is a lack of criteria to which type of SDF should be recommended
In general, the methods for assessing sperm DNA damage can be grouped into three categories, namely: (I) assays that measure DNA fragmentation by incorporating DNA probes or modified nucleotides at the site of breaks; (II) assays that measure both the existing breaks and those generated after DNA denaturation; and (III) assays that indirectly measure the level of chromatin compaction, i.e., nucleus decondensation (116). While TUNEL belongs to the first group, SCSA, SCD, and Comet are examples of tests from the second group. In contrast, aniline blue and toluidine blue are methods that assess chromatin compaction (decondensation) rather than SDF (116).
Mostly, SDF refers to SS-DB or DS-DB and is predominantly associated with OS whereas sperm nucleus decondensation (SND) relates to defects in chromatin compaction (e.g., protamine mispackage via defective DNA-protein crosslinking), which are intrinsically associated with the later stages of spermatogenesis. A synergistic action among different effectors can contribute to the accumulation of DNA damage. As such, defective chromatin compaction can make the DNA more vulnerable to damage by ROS, and as a consequence SDF may ensue (116).
Due to these distinct assays’ characteristics, the results obtained from one method do not necessarily match those provided by a different test, especially if the methods being compared assess SDF and SND (16). Notwithstanding, there seems to be a fair correlation among the three mostly commonly used SDF tests, namely, SCSA, TUNEL and SCD (133,163,164), albeit the evidence is not unequivocal (17). SDF measurement with flow cytometer has been advocated as more precise and reliable (63,78), as the technique allows thousands of cells to be analyzed in a relatively short time compared with the use of optical/fluorescence microscopy. Moreover, the ability to evaluate a large cell number is another means to control inter- and intra-laboratory variations in test results (127,156,158). At present, however, suggesting one specific assay in preference over another does not seem to be in the best interest of clinicians and patients alike until a gold standard method is established.
Since the ideal method to measure SDF is still to be determined, any decision to consider SDF testing should take into account the limitations of testing methods and the possible benefits for clinical outcomes (117). In fact, the different type of SDF detected by each assay may be complementary to each other in distinct clinical settings (107). Nevertheless, it is essential that a reliable SDF assay with a validated threshold and performed by a skilled technician be used (10,16).
SDF lacks clear cut-off values
Although no definite threshold of DNA damage beyond which a seminal sample can be considered pathological has been agreed upon, cut-off values have been reported for SDF testing with regards to pregnancy prediction and infertility risk (112). For instance, recent data shows that SDF test by SCD, when used in a fertility clinic setting, has a sensitivity of 80.8% and specificity of 86.1% with the SDF cutoff of 26.1%, and a prevalence ratio of 2.84 for the occurrence of male infertility (165). Moreover, the benchtop flow cytometer TUNEL assay has been recently standardized and validated on semen samples obtained from 95 fertile controls and 261 infertile men (156). In this study, a SDF cutoff value of 16.8% was found to have a specificity of 91.6% and a positive predictive value of 91.4% in distinguishing infertile men from controls.
Along the same lines, SCD test was found to have a sensitivity of 86.2% and a negative predictive value (NPV) of 72.7% (P=0.02) in predicting successful ART treatment when the cutoff value of 25.5% was utilized (166). Likewise, a SCSA cutoff value of 30% was found to carry a significant predictive power to the likelihood of pregnancy both in vivo and after ART, where patients with a SDF <30% were 7.1 times [95% confidence interval (CI), 3.37–14.91] more likely to achieve a pregnancy in vivo and ~2.0 times (95% CI, 1.10–2.96) more likely to achieve pregnancy after ART (167). Another recent study by Rilcheva et al. (168) utilized the SCSA assay to investigate the influence of SDF on the pregnancy outcome of 531 couples undergoing autologous ICSI (n=416), donation ICSI (n=39) and IUI (n=71). Using a cutoff value of 27%, the authors reported a statistically significant negative relationship between SDF and pregnancy outcome with IUI (χ2=6.87; P<0.05), and a positive relationship between SDF and pregnancy loss after IUI (t-test =1.58; P<0.05) and ICSI (OR =5.65; 95% CI: 4.32–7.11; P<0.05). The authors concluded that infertile men should be evaluated with SDF in addition to routine semen analysis and suggested that when the result exceeds 27%, patients should be offered ICSI at an earlier stage.
Collectively, the threshold range of 25–30% by SCSA or SCD seems valid for placing men into a statistical probability of longer time to achieve natural pregnancy, low odds of pregnancy by IUI and conventional IVF, and a higher risk of miscarriages, both natural or assisted (112,169). However, it is important is to note that having a SDF rate of 30% does not mean that 70% of remaining spermatozoa have entirely normal chromatin (72). The methods to evaluate chromatin integrity measure the percentage of cells with fragmented DNA/chromatin decondensation only and are based on the idea that the greater the fragmentation/decondensation rate, the greater the chance that the sperm population is pathological. It has been suggested that part of the remaining sperm may be already compromised as regards to DNA integrity, but not yet crossed the threshold detectable by the assay (170). High SDF probably represents the tip of an iceberg (72,127,170).
It is therefore crucial that clinicians be judicious when interpreting test results and using this information to predict reproductive outcomes. A high SDF result should be read as a general indicator of poor semen quality instead of an absolute value of abnormal sperm (57,130). Along these lines, nomograms taking into account various levels of SDF and female factors have been suggested as a means to better predict the reproductive outcomes (57). Consideration of female factors is important as the presence of SS-DB may be repaired by oocyte repair machinery (171), thereby preventing the adverse consequences of SDF. However, the oocyte has a limited capacity of repairing SDF (172) and importantly, not all types of sperm DNA breaks are repairable (173). Given the multitude of confounding factors, various cut-off values may be required to ensure satisfactory performance of the test in distinct clinical scenarios (93,127).
SDF tsting does not identify the type (nature of lesion) and site of DNA breaks
Currently, existing SDF methods are limited in revealing the kind of DNA damage and the location of break. However, as discussed above, TUNEL, SCSA, SCD test and Comet determine the presence of DNA breaks whereas aniline blue and toluidine blue the degree of chromatin compaction (decondensation) (116). None of them, however, can discriminate if the lesion affects coding or non-coding DNA domains. Since only about 1.5% of the genome encodes DNA sequences capable of producing proteins (10), it has been argued that lesions affecting non-coding sites may not be of clinical significance (33). However, the remaining genome may have specific functions ranging from various structural roles (highly repeated sequences) to direct regulation of gene expression or indirect gene expression control through epigenetic changes (174-176). Moreover, DS-DBs, especially if abundant within the nucleus, can trigger cell cycle blocking mechanisms that result in cell death (177,178). Cell death can also occur from unrepaired or erroneously repaired DNA lesions that lead to protein production deficiencies or even chromosomal abnormalities (177).
Collectively, apoptotic induction, erroneous DNA repair, and the presence of chromosomal alterations can cause cell death, and these may occur regardless of the location of the initial DNA lesions or whether or not the DNA domain has an informative capacity (10). Nonetheless, the stratification of DNA damage into testicular or post-testicular events could help in our understanding of the pathophysiology and development of new treatment strategies (100). Although neither the amount of SDF in each cell—with the exception of Comet assay—nor the exact site of damage is quantified by SDF tests, a positive association between SDF and natural pregnancy/ART/miscarriage has been demonstrated despite the use of a wide variety of testing methods (19). Rather than being a limitation, the inability of SDF testing to assess how much DNA damage exists in each cell is advantageous given the large number of spermatozoa and the highly variable DNA integrity of each spermatozoon (130). As it stands, SDF testing reflects the quality of the DNA package in the entire semen sample, which provides an overall indication of DNA integrity and male fertility (135).
SDF testing is carried out in neat semen and may not reflect the sperm population used in ART
Although further research is needed in this area, the validity of using processed semen samples for SDF testing is currently not warranted. DNA fragmentation index (DFI) of density gradient centrifugation (DGC)-processed sperm measured by SCSA did not predict ART outcome in contrast to neat samples (103). Besides, no association between sperm SCSA DFI after swim-up and fertilization, implantation and pregnancy rates could be demonstrated in another study (179). Also, DGC has been reported to result in increased SDF especially when higher centrifugation force, longer duration and Percoll gradients were used (180,181). Furthermore, in a study investigating the dynamics of SDF by SCD and its implication on embryo development and pregnancy rate in couples undergoing ICSI, it was observed that the likelihood of pregnancy was decreased by 5.9% for every 1-unit increase in SDF observed after 12 h of incubation (182). These observations suggest that SDF results after sperm processing may increase as a function of factors such as incubation period and temperature, thus making its value questionable for pregnancy prediction in the ART setting.
Threats
Professional societies have not endorsed the use of SDF testing in daily practice
The literature is rich in systematic reviews and meta-analyses reporting on a positive association between elevated SDF and infertility and ART outcomes. However, variation in SDF assays, SDF thresholds and differences in study populations have resulted in mixed conclusions concerning the clinical utility of SDF testing (83,183). Consequently, professional societies, such as the American Society for Reproductive Medicine (ASRM), the American Urological Association (AUA), the European Association of Urology (EAU), and the National Institute of Clinical Excellence (NICE), have not recommended SDF testing for routine clinical evaluation (74,114,184-187).
However, new evidence has been generated steadily after the publication of these guidelines. Studies reporting a significant detrimental relationship between SDF and clinical varicocele (11,187,188), unexplained infertility (189-192), and outcomes of ART (18,193,194) were published recently. Furthermore, new data emerged concerning the potential benefit of using testicular in preference over ejaculated sperm for ICSI in cases of high SDF (195-199), thus stressing the need for further updates in the clinical recommendations of the societies mentioned above. As CPGs are evolving documents, a timely review and update are highly expected.
Equally important is to consider that in addition to the sound guidance provided by CPGs, the application of a test should be weighed by the magnitude of benefit it can bring to a couple (103). Although it is unlikely that the dynamic interaction among multiple confounding factors, both in natural conception and ART, can be confirmed by a single test (109), SDF test in the clinical setting may aid in: (I) identification of the possible underlying etiology in couples who are otherwise classified as unexplained or idiopathic infertility; (II) monitoring of treatment outcomes of either empirical or new treatment modalities; (III) stratification of patients to receive more targeted treatment for SDF; and (IV) avoidance of unnecessary workup and wastage of precious time and money in unproductive treatments (93).
SDF testing is expensive and not reimbursed by insurance companies
The cost of SDF testing is approximately 170±123 USD (18), which remains an important factor limiting the routine use of SDF testing. However, cost is also a major drawback to all other fertility related therapeutic modalities and has been recognized to add significant handicap on infertile couples whose fertility treatments are usually not covered by medical insurance (200). SDF testing represents a fraction of the cost of other fertility treatments such as ART procedures or surgical varicocele ligation (112). A major aim of the CPG by Agarwal et al. (20) was to elucidate the circumstances where SDF testing would be most influential on treatment decisions, therefore, with a possible significant impact on the overall treatment cost.
The use of SDF testing in the right clinical scenario can aid in recommending an ART procedure or treatment that is associated with the highest likelihood of pregnancy, thereby eliminating unnecessary cost from less successful therapeutic modalities (95). In one study evaluating the use of SDF testing and testicular sperm in preference over ejaculated sperm for ICSI in men with high SDF in semen, the number needed to treat by testicular compared to ejaculated sperm to obtain an additional live birth per fresh transfer cycles was 4.9 (95% CI: 2.8–16.8) (195). This study suggested that we could avoid one out of five oocyte retrievals to obtain an additional live birth if we used SDF testing in the aforementioned clinical scenario (92).
Besides, the SDF result can help in selecting patients who are most likely to benefit from varicocele ligation eliminating unnecessary surgery. It seems sound that any step that can potentially improve natural pregnancy or ART outcomes will likely to pose a financial benefit to both the couple and society. A more comprehensive workup of male factors by incorporation of SDF testing is certainly an attractive and economical option. The cost of SDF testing will probably be justified since the test results reflect treatment outcome (95).
Lastly, the question of whether or not doctors should ask patients to spend more money to obtain a diagnosis of the status of DNA damage in the sperm as a tool for both the patient and clinician to decide on a course of treatment should be put in the right perspective (68). Given the evidence indicating that SDF is associated with reproductive health issues in the male and the embryo, the question we as individuals and as a medical community must ultimately consider is if we are providing the best care to the infertile couple and the offspring by ignoring the “health” of the sperm (61).
Opportunities
More research has to be conducted to clarify existing gaps and refine SDF as a valid lab method
The decision about the broad applicability of a clinical test is a delicate balance among numerous factors including cost-effectiveness and patient-centeredness. Currently, there exist gaps to be filled concerning SDF testing as a laboratory method, as discussed above. However, many drawbacks perceived by infertility specialists, such as poor validation, low precision, and low accuracy seem to result from a lack of careful appreciation of the emerging evidence concerning the enhanced performance of several test methods. The practice recommendations by Agarwal et al. is the first step forward to bridge the gap between research and clinical practice in promoting SDF testing. As the intended purpose of the CPG (20) was to identify the proper indications of SDF testing, meaningful refinements of its practical methods and diagnostic thresholds can be achieved further (115).
Part 2: SWOT analysis of clinical utility of SDF in specific clinical scenarios
Strengths
The CPG is timely to guide urologists and infertility specialists in requesting SDF tests in proper scenarios
Little information is available to give clinicians guidance according to which SDF testing should be requested (25). The CPG by Agarwal and colleagues (20) is the first attempt to provide this urgently needed practical orientation. It includes clear situations in which SDF testing can be used to benefit patients, with practical and easy-to-understand supportive information (24-26,30,33,54,55,57,61,69,81). The guideline also points out that SDF testing should not be regarded as the gold standard test in the male fertility assessment. Furthermore, SDF testing was not proposed as a replacement for routine semen analysis or to be used indiscriminately in all cases. Assessment of SDF should rather be a tool to provide physicians additional information that can influence their decision of how to counsel and manage their patients (89).
The four case scenarios cover the spectrum of difficult clinical decisions that most fertility specialists encounter in clinical practice. The evidence-based recommendations are extremely valuable for assessment of male subfertility and couples undergoing ART
Despite the availability of numerous studies exploring the impact of SDF on male fertility and reproductive outcomes, an understanding of the clinical utility of such an important test was still lacking. Foremost among all strengths of Agarwal et al.’s CPG is the scope of testing suggested (61). Although evidence is not available at this time to recommend routine screening of all men evaluated at a fertility clinic, the use of SDF testing in specific clinical scenarios is evidence-based and should be implemented in ART clinics not currently employing such assays. In brief, Agarwal et al. provided clear, evidence-based guidelines that should facilitate practical implementation of SDF testing in the clinic with the objective of not only improving ART success rates, but more importantly to improve the health of the father and the offspring (54,61,66).
There is a common belief that SDF is untreatable. The guideline clarifies this issue and provides evidence-based guidance for interventions
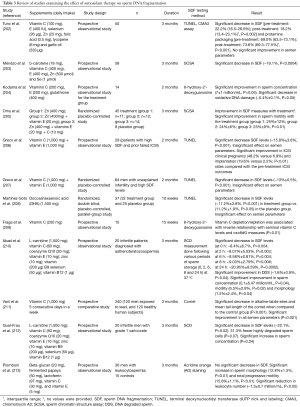
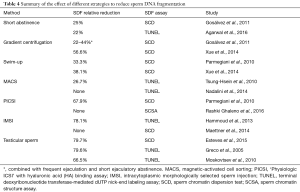
Although the association between SDF and natural pregnancy/ART outcomes has been extensively reported, the lack of effective treatment for high SDF is a common critique of SDF testing (62,120). Despite the common belief that SDF is untreatable, objective evidence indicates that treatment options are available. Among interventions shown to improve sperm DNA integrity, varicocele repair (201) and oral antioxidant therapy can alleviate SDF and improve the chances of establishing a pregnancy (132,202-213) (Table 3). Moreover, modifiable lifestyle factors such as smoking, obesity, and occupational exposure have been associated with high rates of SDF making them potential targets for interventions (128,214-216). It is suggested that correction of underlying factors can alleviate SDF and potentially enable natural conception or allow the use of less complex ART methods (135). If ICSI is to be used, lower miscarriage rates are anticipated after treatment of the conditions causing SDF. For this, a clinical evaluation of the infertile male is essential to identify the causes of infertility and allow treatment of the affected men aiming at reducing SDF. Lastly, laboratory sperm selection techniques and the use of testicular sperm represent alternatives to reduce the risk of using sperm with high SDF for ICSI, with the latter being the most attractive approach given it provides the highest reduction in SDF rates and LBR by ICSI (92,195,197,217-229) (Table 4).
Full table
Full table
A CPG such as the one presented by Agarwal and colleagues (20) is intended to provide clinicians and other healthcare practitioners useful information to enhance the quality of care deliverable to patients (113). It is also designed to discourage potentially harmful or ineffective interventions (84). However, the complexity of the human reproductive system cannot be completely covered by any guideline. The decision to perform a particular investigation, e.g., SDF testing, and to intervene accordingly should be individualized for every couple. The management strategy depends on factors such male and female age, duration of infertility, financial issues as well as couples’ willingness to pursue natural or assisted conception. The primary objective of the CPG under discussion was to translate the best evidence into practice and provide a framework of standardized care while maintaining clinical autonomy and physician judgment (84).
Weaknesses
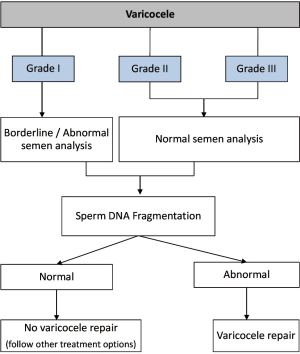
Clinical scenario 1 (varicocele with normal/borderline semen parameters): there is no evidence that SDF alone can predict improvements in semen parameters or affect pregnancy outcomes after varicocele repair
Among infertile men with clinical varicocele, elevated SDF is observed in approximately 50% and 60% of those with normal and abnormal semen parameters, respectively (128,230). Furthermore, 70–90% of patients show improvement in SDF levels after varicocele repair (231,232). Reduction in SDF after surgery is shown to be more common in men who have a concomitant improvement in conventional semen parameters (230). Also, postoperative SDF rates are lower in men able to impregnate their partners naturally or by ART than those who are not (187,230). Notwithstanding, the presence of normal SDF levels in infertile men with clinical varicocele suggests that some patients may have scavenging mechanisms that counteract the effect of excessive ROS as causative of high DNA damage (14).
Although further studies are required, these observations provide some evidence that improvements in sperm DNA integrity after varicocele repair translate into higher pregnancy rates. However, the argument that the usefulness of SDF as a single test needs to be demonstrated is probably flawed given the interaction among multiple factors in the reproductive system (233). SDF testing in providing an assessment of the genetic content of males with varicocele should be considered complementary to conventional semen analysis. As with all diagnostic tests, care must be taken only to utilize them when the results directly affect clinical decision-making. SDF in the context of varicocele should be primarily reserved for the cases in which treatment is not warranted by itself, as depicted in Figure 4.
Clinical scenario 2 (unexplained infertility/recurrent pregnancy loss/IUI failure): little data is available to support the usefulness of screening for SDF
Elevated SDF (DFI >20–30%) is found in up to 45% of infertile men with unexplained infertility (128,189). Pregnancy rates are reduced among couples with unexplained infertility undergoing IUI when SDF rates are >20% (189). Furthermore, the odds of pregnancy are dramatically decreased (by 7.0 to 8.7-fold) in the general population of infertile couples subjected to IUI with high seminal SDF levels (19,234). Evidence of an association between SDF and recurrent pregnancy loss is also increasing steadily (128,190-192). SDF testing in these scenarios may help clinicians counseling the affected men to take all measures to reduce SDF, including oral antioxidant intake and lifestyle changes, and to consider recommending IVF/ICSI as a means to overcome infertility related to poor sperm DNA integrity (Figure 5).

Clinical scenario 3 (IVF/ICSI failure): controversy exists regarding the predictive value of SDF testing and the association between SDF and ICSI outcomes
Most of the controversies in this scenario are due to misunderstandings which prevented proper interpretation and communication. Whereas SDF testing has been commonly utilized to distinguish couples who will or will not become pregnant naturally or with ART, studies examining test performance have used heterogeneous patient populations and outcome measures, thus complicating their interpretation (83). For SDF to be used as a valid screening test, some conditions should be met. Foremost among all is application of the test in the proper population. For instance, the positive predictive value (PPV) of SDF in the ART setting reflects the probability that a couple who tests positive fails IVF/ICSI. In contrast, the NPV is the probability that a couple who tests negative succeed. By all means, SDF testing alone is not suitable to provide an accurate pregnancy prediction due to the multitude of factors affecting ART outcomes. Moreover, the limitations of the test will become more evident if SDF testing is applied to all comers because the PPV and NPV change with the prevalence of the condition. If the prevalence is low (e.g., men without risk factors for SDF), the PPV will be low, even if specificity and sensitivity are high (235). In contrast, if the prevalence is high (e.g., men with risk factors for SDF), the PPV will be high provided a highly specific cutoff value is chosen. As a matter of fact, it has been shown that the PPV of TUNEL concerning pregnancy in ART is only moderate whereas that of SCD is low despite the high sensitivity (77–85%) and specificity (89–91%) of both tests (83,166,236,237).
The use of SDF as a screening test for pregnancy prediction in a population at low risk for sperm DNA damage is highly problematic as couples can be erroneously categorized as having a higher or lower chance to get pregnant when in fact they have not. Because abnormal SDF levels are seen in only about 30% of men from the general IVF/ICSI population (128,167), these implications will become evident when screening all couples to be subjected to ART, as there will be a high number of false positives results (i.e., successful pregnancy despite high SDF, thus resulting in a low PPV). Therefore, SDF testing will be most useful if applied to couples with high risk for an adverse outcome associated with SDF (e.g., men with risk factors and older partners, and couples with repeat IVF/ICSI failures).
Equally important is the influence of the type of ART on treatment outcomes. The negative association between high SDF and pregnancy rates in conventional IVF (112,192,238), but not ICSI (239), clearly illustrate these points. Since SDF testing concerns the integrity of paternal DNA, the poorer the DNA quality, the more fragile the spermatozoon in its ability to generate a healthy embryo. Therefore, the extent of sperm journey may impact IUI and IVF/ICSI outcomes. Reducing the length of the journey to the oocyte (by conventional IVF) as well as bypassing the process of fertilization (by ICSI) can minimize the extent of sperm DNA damage (63).
Additionally, female factors, such as age and oocyte quality, can modulate the impact of SDF on IVF/ICSI outcomes (118). Results of a retrospective clinical study showed that the live birth and implantation rates during IVF/ICSI in women with reduced ovarian reserve were significantly decreased when SDF exceeded 27.3%. In contrast, clinical pregnancy, live birth, and implantation rates were not affected in women with normal ovarian reserve (194). The oocyte capability to repair, at least to some extent, DNA damage means that semen specimens with low to moderate SDF combined with high-quality oocytes may result in acceptable reproductive outcomes. Lastly, the association between high SDF and increased miscarriage after both IVF and ICSI (19,240) suggest that the outcome measured also matters. The negative impact of SDF is more often expressed later at the implantation stage and onwards (late paternal effect) (101,241,242), which may explain why the risk of miscarriage is increased in the face of high SDF whereas clinical pregnancy rates may not be compromised (195).
As far as remedies for recurrent miscarriage/repeated IVF/ICSI failure related to high SDF are concerned, SDF results may guide clinicians as well. Patients can be offered any means to decrease SDF (if a correctable cause is identified) or ICSI with testicular sperm. The latter is based on the increasing evidence indicating that testicular sperm have lower SDF levels than ejaculated sperm and result in superior ICSI outcomes (23,85,125,195,199) (Figure 5).
Clinical scenario 4 (borderline abnormal or normal conventional semen analysis with life-style risk factors for SDF): there is insufficient evidence that lifestyle modification will result in resolution of SDF or improve fertility outcomes
At first glance, the clinical utility of SDF seems to be least convincing in patients with lifestyle risk factors, especially when no other reversible reasons for high SDF are detected (56,90). However, the negative impact of such risk factors on SDF has been consistently reported by several studies [reviewed by Esteves et al. (128)]. Furthermore, recent data indicate that a healthy dietary pattern and lifestyle decrease SDF (215,216). Additionally, evidence suggests that oral antioxidant therapy improve sperm DNA integrity and translate in better changes for establishing a pregnancy using ART (132) (Table 3).
Although further research is warranted to confirm the role of lifestyle changes concerning sperm DNA integrity, and how these changes may translate into better reproductive outcomes, information provided by SDF testing gives solid grounds for implementing lifestyle changes as well as monitoring patient compliance in health prevention programs. Knowledge of the SDF status can be used to strengthen patient counseling and allow clinicians to provide a more realistic prognosis of every modality the couple wishes to pursue.
Overall quality of evidence leads to level C recommendation
While all CPGs include recommendations, it is the quality of the available evidence that shapes the strength of recommendations. It is true that Agarwal et al.’s recommendations (20) are primarily based on levels B and C evidence and that more clinical data should be attained to support their advice further. However, CPGs issued by other societies such as the AUA, EAU, and the ASRM have also synthesized their recommendations largely based on studies of moderate to low quality (5,184-186,243), thus reflecting the limited evidence available in other areas of infertility as well. Nevertheless, the driving force of all involved in contributing guidelines, including Agarwal and his peers, is in translating the best evidence available into practice to serve as a framework for standardized care while maintaining physician autonomy. The existent shortcoming of SDF testing should not refrain physicians to take full advantage of its clinical benefits provided the data supporting that specific test be made clear to the patient.
Threats
Specialty societies and National Institutes do not recommend the use of DNA fragmentation in the routine workup of the infertile male patient
The lack of sufficient high-grade evidence in support of the application of SDF testing in the clinical scenarios discussed above is a criticism often heard (131). Moreover, despite many alternatives to treat patients with high SDF, including oral antioxidants, varicocele ligation, frequent ejaculation, and testicular sperm extraction, good clinical evidence is still lacking to support the routine use of these alternatives. As such, guidelines from various professional societies stress the lack of robust evidence to support the clinical utility of SDF testing (131). Furthermore, practices differ globally and in certain regions (e.g., UK) National Institutes for Health neither endorses varicocele treatment for infertility nor the use of SDF testing for infertility diagnosis and management (74,114). Despite that, the potential benefit of SDF testing in daily practice is increasingly being realized. Recognizing the significant efforts from researchers that have moved SDF testing from bench to clinical practice in the twenty-first century, the Society for Translational Medicine (244) has taken a step ahead and fully endorsed the CPG for SDF testing in male infertility (20,245). Also, the society mentioned above supported the publication of a journal supplement entirely dedicated to the topic in one of its official journals -Translational Andrology and Urology-, in which this paper stands, along with a concise practice recommendation for quick clinical reference (246). This initiative, in addition to the bulk of published data since previous male infertility guidelines were issued, must provide enough fuel for a timely review and update of other societies’ guidelines such as the AUA, ASRM, and EAU.
The guideline is not inclusive of all clinical conditions that may benefit from using SDF testing
Case scenarios are commonly used in medical literature to report a particular clinical condition or contextualize a situation hoping for better comprehension (247,248). Although the use of clinical scenarios to illustrate a point may not apply to a slightly different scenario (54), Agarwal and colleagues utilized this method in their CPG (20) to personalize a message giving it a clinical perspective (90). Infertile men with advanced age, diabetes, subclinical infection, and cancer, among others, may benefit from SDF testing as well so that further response to interventional treatments could be monitored (40,63).
Opportunities
More studies have to be conducted to clarify the clinical utility of SDF test results to determine the course of treatment in the presented clinical scenarios and beyond
Well-designed studies with adequate power and standard techniques will be invaluable to refine the clinical utility of SDF testing further. In this article, we scrutinized the practice recommendations and commentaries from experts concerning its utility to identify existing gaps and potential areas for translational research. Some areas for further investigation concerns: (I) the efficacy of varicocelectomy to reduce SDF rates and their impact on pregnancy outcomes; (II) the role of female age and SDF oocyte repair capacity in natural and assisted conception; (III) the clinical utility of SDF to other categories of patients, such as cancer patients exposed to chemo/radiotherapy, and diabetes; (IV) the role of preimplantation genetic screening in cases of ICSI treatment associated to high SDF; (V) cost-effectiveness of testicular sperm and other methods to reduce SDF for ICSI; and (VI) the role of lifestyle interventions to reduce SDF rates and how the effects impact on pregnancy rates.
Conclusions
Understanding the role of SDF in male infertility requires an in-depth analysis of the multiple pathophysiological processes and the theories involved, as well as the examination of different levels of scientific evidence of published studies. Agarwal and colleagues provide a reasonable proposal for integration of SDF testing in the clinic in the ICSI era, despite inherent limitations in drawing an evidence-based clinical guideline. The uniqueness of SWOT analysis offers the possibility of evaluating this CPG on the clinical utility of SDF testing based on clinical scenarios and its accompanying commentaries written by global experts in all possible angles, thus providing rich information for clinicians and researchers alike. With further refinements in SDF testing and additional supporting evidence of its clinical utility, implementation of SDF testing in the clinic may not only increase ART success but more importantly improve the health of both fathers to be and resulting offspring.
Acknowledgements
The authors are indebted to Prof. Ralf Henkel, Department of Medical Bioscience, University of the Western Cape, South Africa, for his critical revision of the manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Thonneau P, Marchand S, Tallec A, et al. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988-1989). Hum Reprod 1991;6:811-6. [Crossref] [PubMed]
- Esteves SC. Novel concepts in male factor infertility: clinical and laboratory perspectives. J Assist Reprod Genet 2016;33:1319-35. [Crossref] [PubMed]
- Esteves SC, Miyaoka R, Agarwal A. An update on the clinical assessment of the infertile male. Clinics (Sao Paulo) 2011;66:691-700. [Crossref] [PubMed]
- Esteves SC. Clinical relevance of routine semen analysis and controversies surrounding the 2010 World Health Organization criteria for semen examination. Int Braz J Urol 2014;40:443-53. [Crossref] [PubMed]
- Esteves SC, Chan P. A systematic review of recent clinical practice guidelines and best practice statements for the evaluation of the infertile male. Int Urol Nephrol 2015;47:1441-56. [Crossref] [PubMed]
- Esteves SC. Clinical management of infertile men with nonobstructive azoospermia. Asian J Androl 2015;17:459-70. [PubMed]
- Hamada A, Esteves SC, Nizza M, Agarwal A. Unexplained male infertility: diagnosis and management. Int Braz J Urol 2012;38:576-94. [Crossref] [PubMed]
- Esteves SC. A clinical appraisal of the genetic basis in unexplained male infertility. J Hum Reprod Sci 2013;6:176-82. [Crossref] [PubMed]
- Lewis SE, Aitken JR, Conner SJ, et al. The impact of sperm DNA damage in assisted conception and beyond: recent advances in diagnosis and treatment. Reprod Biomed Online 2013;27:325-37. [Crossref] [PubMed]
- Gosálvez J, Lopez-Fernandez C, Fernandez JL, et al. Unpacking the mysteries of sperm DNA fragmentation: ten frequently asked questions. J Reprod Biotechnol Fertil 2015;4:1-16. [Crossref]
- Esteves SC, Gosálvez J, López-Fernández C, et al. Diagnostic accuracy of sperm DNA degradation index (DDSi) as a potential noninvasive biomarker to identify men with varicocele-associated infertility. Int Urol Nephrol 2015;47:1471-7. [Crossref] [PubMed]
- Saleh RA, Agarwal A, Nelson DR, et al. Increased sperm nuclear DNA damage in normozoospermic infertile men: a prospective study. Fertil Steril 2002;78:313-8. [Crossref] [PubMed]
- Sakkas D, Alvarez JG. Sperm DNA fragmentation: mechanisms of origin, impact on reproductive outcome, and analysis. Fertil Steril 2010;93:1027-36. [Crossref] [PubMed]
- Agarwal A, Hamada A, Esteves SC. Insight into oxidative stress in varicocele-associated male infertility: part 1. Nat Rev Urol 2012;9:678-90. [Crossref] [PubMed]
- Muratori M, Tamburrino L, Marchiani S, et al. Investigation on the origin of sperm DNA fragmentation: role of apoptosis, immaturity and oxidative stress. Mol Med 2015;21:109-22. [Crossref] [PubMed]
- Esteves SC, Sharma RK, Gosálvez J, et al. A translational medicine appraisal of specialized andrology testing in unexplained male infertility. Int Urol Nephrol 2014;46:1037-52. [Crossref] [PubMed]
- Feijó CM, Esteves SC. Diagnostic accuracy of sperm chromatin dispersion test to evaluate sperm deoxyribonucleic acid damage in men with unexplained infertility. Fertil Steril 2014;101:58-63.e3. [Crossref] [PubMed]
- Majzoub A, Agarwal A, Cho CL, et al. Sperm DNA fragmentation testing: a cross sectional survey on current practices of fertility specialists. Transl Androl Urol 2017;6:S710-9.
- Agarwal A, Cho CL, Esteves SC. Should we evaluate and treat sperm DNA fragmentation? Curr Opin Obstet Gynecol 2016;28:164-71. [Crossref] [PubMed]
- Agarwal A, Majzoub A, Esteves SC, et al. Clinical utility of sperm DNA fragmentation testing: practice recommendations based on clinical scenarios. Transl Androl Urol 2016;5:935-50. [Crossref] [PubMed]
- Bento FC, Esteves SC. Establishing a quality management system in a fertility center: experience with ISO 9001. Medicalexpress 2016;3:M160302. [Crossref]
- Engmann L, Benadiva C, Humaidan P. GnRH agonist trigger for the induction of oocyte maturation in GnRH antagonist IVF cycles: a SWOT analysis. Reprod Biomed Online 2016;32:274-85. [Crossref] [PubMed]
- Esteves SC, Roque M, Garrido N. Use of testicular sperm for intracytoplasmic sperm injection in men with high sperm DNA fragmentation: a SWOT analysis. Asian J Androl 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Cho CL. Commentary: sperm DNA fragmentation testing in action. Transl Androl Urol 2017;6:S647-8.
- Henkel R. Clinical utility of sperm DNA fragmentation testing: a commentary. Transl Androl Urol 2017;6:S632-5.
- Kosgi R. Sperm DNA fragmentation testing - clinical utility. Transl Androl Urol 2017;6:S654-5.
- Arafa M, ElBardisi H. Clinical implication of DNA fragmentation in male infertility. Transl Androl Urol 2017;6:S656-7.
- Tadros NN, Sabanegh E Jr. Commentary on clinical utility of sperm DNA fragmentation testing. Transl Androl Urol 2017;6:S374-6.
- Pandiyan N, Pandiyan R, Raja DR. A perspective on sperm DNA fragmentation. Transl Androl Urol 2017;6:S661-4.
- Gosálvez J. Clinical utility of sperm DNA fragmentation testing: article overview. Transl Androl Urol 2017;6:S532-4.
- Benagiano G, Paoli D, Lombardo F, et al. DNA fragmentation and the ultimate success of a pregnancy. Transl Androl Urol 2017;6:S539-43.
- Turek PJ. Finding the fit: sperm DNA integrity testing for male infertility. Transl Androl Urol 2017;6:S379-80.
- Garrido N, Rivera R, Luján S. Clinical use of sperm DNA fragmentation analysis results, a practical example of how to deal with too much information from the literature in reproductive medicine. Transl Androl Urol 2017;6:S547-8.
- Oehninger S. Sperm DNA fragmentation testing: ready for prime time? Transl Androl Urol 2017;6:S385-8.
- El-Sakka AI. Routine assessment of sperm DNA fragmentation in clinical practice: commentary and perspective. Transl Androl Urol 2017;6:S640-3.
- Menezo Y, Clement P, Amar E. Evaluation of sperm DNA structure, fragmentation and decondensation: an essential tool in the assessment of male infertility. Transl Androl Urol 2017;6:S553-6.
- Yovich JL, Keane KN. Assessing the male in fertility clinics—men undervalued, undermanaged and undertreated. Transl Androl Urol 2017;6:S624-8.
- Hsiao W. Sperm DNA fragmentation: indication and uses. Transl Androl Urol 2017;6:S392-3.
- Khandwala YS, Eisenberg ML. Practical Applications of Sperm DNA Fragmentation Testing and its Role in Infertility. Transl Androl Urol 2017;6:S397-8.
- Muratori M, Baldi E. Some relevant points on sperm DNA fragmentation tests. Transl Androl Urol 2017;6:S560-3.
- Khalili MA, Agha-Rahimi A. Use of sperm DNA fragmentation test in ART setting: is it a promising diagnostic test? Transl Androl Urol 2017;6:S673-4.
- Tesarik J, Galán-Lázaro M. Clinical scenarios of unexplained sperm DNA fragmentation and their management. Transl Androl Urol 2017;6:S566-9.
- Basar MM, Kahraman S. Clinical utility of sperm DNA fragmentation testing: practice recommendations based on clinical scenarios. Transl Androl Urol 2017;6:S574-6.
- Franco JG Jr. Sperm DNA fragmentation. Transl Androl Urol 2017;6:S516-8.
- Borini A, Tarozzi N, Nadalini M. Sperm DNA fragmentation testing in male infertility work-up: are we ready? Transl Androl Urol 2017;6:S580-2.
- Vandekerckhove F. Guidelines on sperm DNA fragmentation testing. Transl Androl Urol 2017;6:S586-7.
- Rey RA. Commentary on sperm DNA fragmentation testing clinical guideline. Transl Androl Urol 2017;6:S522-4.
- Mirzazadeh M, Sadri-Ardekani H. Implications of ejaculatory sperm DNA fragmentation on male infertility management. Transl Androl Urol 2017;6:S402-4.
- Pool TB. Sperm DNA fragmentation: the evolution of guidelines for patient testing and management. Transl Androl Urol 2017;6:S409-11.
- Glina S. Sperm DNA fragmentation testing: when and why? Transl Androl Urol 2017;6:S504-5.
- Amiri-Yekta A, Arnoult C, Ray PF. Measure of sperm DNA fragmentation (SDF): how, why and when? Transl Androl Urol 2017;6:S588-9.
- Drevet JR. Sperm DNA integrity testing: a valuable addition to the tool box of infertility clinicians. Transl Androl Urol 2017;6:S590-1.
- Durairajanayagam D. Commentary: the value of testing sperm DNA fragmentation in infertile men. Transl Androl Urol 2017;6:S678-80.
- Lynne CM, Brackett NL. A guide to sperm DNA fragmentation testing. Transl Androl Urol 2017;6:S414-5.
- Mathur PP. Recognizing sperm DNA fragmentation testing in clinical evaluation of male fertility. Transl Androl Urol 2017;6:S681-2.
- Fraczek M, Kurpisz M. Is the sperm DNA status the best predictor of both natural and assisted conception? Transl Androl Urol 2017;6:S594-6.
- Jarvi KA. Is sperm DNA fragmentation testing ready for prime time? Transl Androl Urol 2017;6:S419-21.
- Johnson D, Sandlow J. Sperm DNA fragmentation testing: proceed with care. Transl Androl Urol 2017;6:S425-7.
- Kadioglu A, Ortac M. The role of sperm DNA testing on male infertility. Transl Androl Urol 2017;6:S600-3.
- Marmar JL. Is testing of sperm DNA fragmentation (SDF) ready for the basic work-up of male infertility? Transl Androl Urol 2017;6:S437-9.
- Carrell DT, Hotaling J. Using sperm testing to improve patient and offspring health: rational, evidence-based care of the infertile male in the ART clinic. Transl Androl Urol 2017;6:S443-5.
- Tatem AJ, Brannigan RE. The role for sperm DNA damage testing in 2017. Transl Androl Urol 2017;6:S448-9.
- Christensen P, Humaidan P. Testing of sperm DNA damage and clinical recommendations. Transl Androl Urol 2017;6:S607-9.
- Cunha-Filho JS. Evidence based sperm DNA fragmentation. Transl Androl Urol 2017;6:S527-8.
- Mehta A. Pros and cons of sperm DNA fragmentation testing: weighing the evidence. Transl Androl Urol 2017;6:S453-4.
- Herati AS, Lamb DJ. Frontiers in sperm function testing: DNA fragmentation analysis shows promise. Transl Androl Urol 2017;6:S457-8.
- Veeramachaneni DN. Utility of testing sperm DNA fragmentation: an all-in-one diagnostic tool to address a multi-pronged clinical problem. Transl Androl Urol 2017;6:S462-4.
- Ward WS. Eight tests for sperm DNA fragmentation and their roles in the clinic. Transl Androl Urol 2017;6:S468-70.
- Samplaski MK. A few more fragments: putting sperm DNA integrity testing into clinical practice. Transl Androl Urol 2017;6:S473-5.
- Ahmad G. Clinical utility of sperm DNA fragmentation testing: a requisite to infertility practice. Transl Androl Urol 2017;6:S685-7.
- Kovac JR. Practical considerations for DNA fragmentation testing in the management of male fertility. Transl Androl Urol 2017;6:S479-80.
- Hallak J. Utility of sperm DNA fragmentation testing in different clinical scenarios of male reproductive abnormalities and its influence in natural and assisted reproduction. Transl Androl Urol 2017;6:S509-12.
- Dada R. Sperm DNA damage diagnostics: when and why. Transl Androl Urol 2017;6:S691-4.
- Potdar N. Application of sperm DNA fragmentation test in clinical setting. Transl Androl Urol 2017;6:S613-4.
- Tandon S. Sperm DNA fragmentation testing: where we stand in 2017. Transl Androl Urol 2017;6:S697-8.
- Varghese AC, Tan G, Chan P, et al. Clinical usefulness of sperm DNA fragmentation testing. Transl Androl Urol 2017;6:S484-7.
- O’Flaherty C. The quest of finding the perfect spermatozoon. Transl Androl Urol 2017;6:S491-2.
- Evenson DP. Evaluation of sperm chromatin structure and DNA strand breaks is an important part of clinical male infertility assessment. Transl Androl Urol 2017;6:S495-500.
- Malhotra V. Should sperm DNA fragmentation testing be routinely used in assessing male infertility? Transl Androl Urol 2017;6:S699-701.
- Ferlin A. Sperm DNA fragmentation testing as a diagnostic and prognostic parameter of couple infertility. Transl Androl Urol 2017;6:S618-20.
- Harlev A. Infertility, recurrent pregnancy loss and sperm DNA fragmentation, have we found the missing link? Transl Androl Urol 2017;6:S704-6.
- Majzoub A, Agarwal A, Esteves SC. Elucidating the clinical indications of sperm DNA fragmentation in male infertility. Transl Androl Urol 2017;6:S658-60.
- Esteves SC, Majzoub A, Agarwal A. The problem of mixing ‘apples and oranges’ in meta-analytic studies. Transl Androl Urol 2017;6:S412-3.
- Esteves SC, Agarwal A, Majzoub A. Unraveling the utility and limitations of clinical practice guidelines. Transl Androl Urol 2017;6:S506-8.
- Esteves SC, Majzoub A, Agarwal A. More good than harm should be expected when Testi-ICSI is applied to oligozoospermic men with post-testicular sperm DNA fragmentation. Transl Androl Urol 2017;6:S381-4.
- Majzoub A, Agarwal A, Esteves SC. Sperm DNA fragmentation: a key player in decision making. Transl Androl Urol 2017;6:S394-6.
- Esteves SC, Agarwal A, Majzoub A. Sperm DNA fragmentation test results reflect the overall quality of the whole semen specimen. Transl Androl Urol 2017;6:S592-3.
- Esteves SC, Agarwal A, Majzoub A. Best practice statements are not intended to dictate an exclusive course of management. Transl Androl Urol 2017;6:S683-4.
- Majzoub A, Agarwal A, Esteves SC. Sperm DNA fragmentation in clinical practice. Transl Androl Urol 2017;6:S544-6.
- Majzoub A, Agarwal A, Esteves SC. The value of sperm DNA fragmentation testing in real-life clinical presentations. Transl Androl Urol 2017;6:S416-8.
- Majzoub A, Agarwal A, Esteves SC. Sperm DNA fragmentation: a rationale for its clinical utility. Transl Androl Urol 2017;6:S455-6.
- Esteves SC, Agarwal A, Majzoub A. Comparison of strategies to reduce sperm DNA fragmentation in couples undergoing ICSI. Transl Androl Urol 2017;6:S570-3.
- Agarwal A, Cho CL, Majzoub A, et al. Call for wider application of sperm DNA fragmentation test. Transl Androl Urol 2017;6:S399-401.
- Agarwal A, Cho CL, Majzoub A, et al. Expanding treatment paradigm of high sperm DNA fragmentation. Transl Androl Urol 2017;6:S450-2.
- Agarwal A, Cho CL, Majzoub A, et al. Sperm DNA fragmentation testing is the safe and economical way to go. Transl Androl Urol 2017;6:S446-7.
- Majzoub A, Agarwal A, Esteves SC. Sperm DNA fragmentation for the evaluation of male infertility: clinical algorithms. Transl Androl Urol 2017;6:S405-8.
- Agarwal A, Cho CL, Majzoub A, et al. Development of targeted therapeutic strategies and refinement of sperm DNA fragmentation testing. Transl Androl Urol 2017;6:S610-2.
- Majzoub A, Agarwal A, Esteves SC. Sperm DNA fragmentation testing in patients with subclinical varicocele: is there any evidence? Transl Androl Urol 2017;6:S459-61.
- Agarwal A, Cho CL, Esteves SC, et al. Current limitation and future perspective of sperm DNA fragmentation tests. Transl Androl Urol 2017;6:S549-52.
- Agarwal A, Cho CL, Esteves SC, et al. Sperm DNA fragmentation testing is on the right track. Transl Androl Urol 2017;6:S389-91.
- Esteves SC, Agarwal A, Majzoub A. Live birth must be the primary reproductive endpoint in IVF/ICSI studies evaluating sperm DNA fragmentation testing. Transl Androl Urol 2017;6:S564-7.
- Agarwal A, Cho CL, Majzoub A, et al. Risk factors associated with sperm DNA fragmentation. Transl Androl Urol 2017. [Epub ahead of print].
- Agarwal A, Cho CL, Esteves SC, et al. Implication of sperm processing during assisted reproduction on sperm DNA integrity. Transl Androl Urol 2017;6:S583-5.
- Majzoub A, Agarwal A, Esteves SC. Understanding sperm DNA fragmentation. Transl Androl Urol 2017;6:S535-8.
- Agarwal A, Cho CL, Majzoub A, et al. Drawbacks of the current practice. Transl Androl Urol 2017;6:S529-31.
- Agarwal A, Cho CL, Esteves SC, et al. All-round approach in diagnosis. Transl Androl Urol 2017;6:S465-7.
- Agarwal A, Cho CL, Majzoub A, et al. From bench to clinic. Transl Androl Urol 2017;6:S471-2.
- Agarwal A, Cho CL, Esteves SC, et al. Development of treatment strategies in men with vulnerable sperm. Transl Androl Urol 2017;6:S476-8.
- Esteves SC, Majzoub A, Agarwal A. Despite limitations, SDF testing provides unique information complementary to but distinct from semen analysis results. Transl Androl Urol 2017;6:S377-8.
- Esteves SC, Majzoub A, Agarwal A. The importance of quality control and quality assurance in SDF testing. Transl Androl Urol 2017;6:S604-6.
- Esteves SC, Majzoub A, Agarwal A. Expanding our understanding of clinical laboratory testing in male infertility patients. Transl Androl Urol 2017;6:S440-2.
- Majzoub A, Agarwal A, Esteves SC. Insights on the predictive accuracy of the sperm DNA fragmentation tests on male infertility. Transl Androl Urol 2017;6:S644-6.
- Agarwal A, Cho CL, Majzoub A, et al. The missing piece in management of infertile couple— clinical andrology. The missing piece in management of infertile couple—clinical andrology. Transl Androl Urol 2017;6:S481-3.
- Agarwal A, Cho CL, Majzoub A, et al. Is National Institute of Clinical Excellence (NICE) guideline a nice guideline? Transl Androl Urol 2017;6:S615-7.
- Majzoub A, Agarwal A, Esteves SC. Sperm DNA fragmentation: laboratory and clinical aspects. Transl Androl Urol 2017;6:S675-7.
- Esteves SC, Agarwal A, Majzoub A. The complex nature of the sperm DNA damage process. Transl Androl Urol 2017;6:S557-9.
- Esteves SC, Majzoub A, Agarwal A. Technical aspects of sperm DNA fragmentation testing, methods to select sperm with low DNA fragmentation, and usefulness of redox potential measurement in male infertility. Transl Androl Urol 2017;6:S636-9.
- Agarwal A, Cho CL, Majzoub A, et al. The role of female factors in the management of sperm DNA fragmentation. Transl Androl Urol 2017;6:S488-90.
- Esteves SC, Majzoub A, Agarwal A. Integrating surgical and clinical andrology is essential to improve the quality of care delivered to infertile couples. Transl Androl Urol 2017;6:S629-31.
- Agarwal A, Cho CL, Majzoub A, et al. Restoration of fertility potential via targeted treatment approach. Transl Androl Urol 2017;6:S493-4.
- Esteves SC, Agarwal A, Majzoub A. An evidence-based perspective on the role of sperm chromatin integrity and sperm DNA fragmentation testing in male infertility. Transl Androl Urol 2017;6:S665-72.
- Agarwal A, Cho CL, Esteves SC, et al. Reactive oxygen species and sperm DNA fragmentation. Transl Androl Urol 2017;6:S695-6.
- Agarwal A, Cho CL, Esteves SC, et al. The price and value of sperm DNA fragmentation tests. Transl Androl Urol 2017;6:S597-9.
- Cho CL, Agarwal A, Majzoub A, et al. Future direction in sperm DNA fragmentation testing. Transl Androl Urol 2017;6:S525-6.
- Cho CL, Agarwal A, Majzoub A, et al. Use of sperm DNA fragmentation testing and testicular sperm for intracytoplasmic sperm injection. Transl Androl Urol 2017;6:S688-90.
- Cho CL, Agarwal A, Majzoub A, et al. It is high time for clinical application of sperm DNA fragmentation testing. Transl Androl Urol 2017;6:S577-9.
- Cho CL, Agarwal A, Majzoub A, et al. Sperm DNA fragmentation testing reveals the overall quality of a semen sample. Transl Androl Urol 2017;6:S513-5.
- Esteves SC, Majzoub A, Agarwal A. Further evidence supports the clinical utility of sperm DNA fragmentation testing in male infertility workup and assisted reproductive technology. Transl Androl Urol 2017;6:S428-36.
- Cho CL, Agarwal A, Majzoub A, et al. One of the many missing links between infertility and sperm DNA fragmentation. Transl Androl Urol 2017;6:S707-9.
- Cho CL, Agarwal A, Majzoub A, et al. The correct interpretation of sperm DNA fragmentation test. Transl Androl Urol 2017;6:S621-3.
- Cho CL, Agarwal A, Majzoub A, et al. The debate on sperm DNA fragmentation test goes on. Transl Androl Urol 2017;6:S702-3.
- Majzoub A, Agarwal A, Esteves SC. Antioxidants for elevated sperm DNA fragmentation: a mini review. Transl Androl Urol 2017;6:S649-53.
- Majzoub A, Agarwal A, Esteves SC. Sperm DNA fragmentation: overcoming standardization obstacles. Transl Androl Urol 2017;6:S422-4.
- Hamada AJ, Esteves SC, Agarwal A. A comprehensive review of genetics and genetic testing in azoospermia. Clinics (Sao Paulo) 2013;68 Suppl 1:39-60. [Crossref] [PubMed]
- Zini A, Bach PV, Al-Malki AH, et al. Use of testicular sperm for ICSI in oligozoospermic couples: how far should we go? Hum Reprod 2017;32:7-13. [PubMed]
- Esteves SC, Zini A, Aziz N, et al. Critical appraisal of World Health Organization's new reference values for human semen characteristics and effect on diagnosis and treatment of subfertile men. Urology 2012;79:16-22. [Crossref] [PubMed]
- Riddell D, Pacey A, Whittington K. Lack of compliance by UK andrology laboratories with World Health Organization recommendations for sperm morphology assessment. Hum Reprod 2005;20:3441-5. [Crossref] [PubMed]
- Alvarez C, Castilla JA, Ramírez JP, et al. External quality control program for semen analysis: Spanish experience. J Assist Reprod Genet 2005;22:379-87. [Crossref] [PubMed]
- Cooper TG, Björndahl L, Vreeburg J, et al. Semen analysis and external quality control schemes for semen analysis need global standardization. Int J Androl 2002;25:306-11. [Crossref] [PubMed]
- Cooper TG, Björndahl L, Vreeburg J, et al. External quality control schemes for semen analysis need global standardization. Int J Androl 2002;25:306-11. [Crossref] [PubMed]
- Alvarez C, Castilla JA, Martinez L, et al. Biological variation of seminal parameters in healthy subjects. Hum Reprod 2003;18:2082-8. [Crossref] [PubMed]
- Agarwal A, Esteves SC, Gupta S, et al. Urology 2016;94:109-10. Author Reply. [Crossref] [PubMed]
- Sakkas D, Urner F, Bizzaro D, et al. Sperm nuclear DNA damage and altered chromatin structure: effect on fertilization and embryo development. Hum Reprod 1998;13:11-9. [Crossref] [PubMed]
- Samplaski MK, Dimitromanolakis A, Lo KC, et al. The relationship between sperm viability and DNA fragmentation rates. Reprod Biol Endocrinol 2015;13:42. [Crossref] [PubMed]
- Smit M, Dohle GR, Hop WC, et al. Clinical correlates of the biological variation of sperm DNA fragmentation in infertile men attending an andrology outpatient clinic. Int J Androl 2007;30:48-55. [Crossref] [PubMed]
- Evenson DP, Jost LK, Baer RK, et al. Individuality of DNA denaturation patterns in human sperm as measured by the sperm chromatin structure assay. Reprod Toxicol 1991;5:115-25. [Crossref] [PubMed]
- Zini A, Kamal K, Phang D, et al. Biologic variability of sperm DNA denaturation in infertile men. Urology 2001;58:258-61. [Crossref] [PubMed]
- Agarwal A, Said TM. Role of sperm chromatin abnormalities and DNA damage in male infertility. Hum Reprod Update 2003;9:331-45. [Crossref] [PubMed]
- Henkel R, Kierspel E, Stalf T, et al. Effect of reactive oxygen species produced by spermatozoa and leukocytes on sperm functions in non-leukocytospermic patients. Fertil Steril 2005;83:635-42. [Crossref] [PubMed]
- Agarwal A, Said TM, Bedaiwy MA, et al. Oxidative stress in an assisted reproductive techniques setting. Fertil Steril 2006;86:503-12. [Crossref] [PubMed]
- Gosálvez J, Coppola L, Fernández JL, et al. Multi-centre assessment of nitroblue tetrazolium reactivity in human semen as a potential marker of oxidative stress. Reprod Biomed Online 2017;34:513-21. [Crossref] [PubMed]
- Hamada A, Esteves SC, Agarwal A. Insight into oxidative stress in varicocele-associated male infertility: part 2. Nat Rev Urol 2013;10:26-37. [Crossref] [PubMed]
- Cho CL, Esteves SC, Agarwal A. Novel insights into the pathophysiology of varicocele and its association with reactive oxygen species and sperm DNA fragmentation. Asian J Androl 2016;18:186-93. [Crossref] [PubMed]
- Esteves SC, Hamada A, Kondray V, et al. What every gynecologist should know about male infertility: an update. Arch Gynecol Obstet 2012;286:217-29. [Crossref] [PubMed]
- Snow-Lisy D, Sabanegh E Jr. What does the clinician need from an andrology laboratory? Front Biosci (Elite Ed) 2013;5:289-304. [Crossref] [PubMed]
- Sharma R, Ahmad G, Esteves SC, et al. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay using bench top flow cytometer for evaluation of sperm DNA fragmentation in fertility laboratories: protocol, reference values, and quality control. J Assist Reprod Genet 2016;33:291-300. [Crossref] [PubMed]
- McEvoy A, Roberts P, Yap K, et al. Development of a simplified method of human semen storage for the testing of sperm DNA fragmentation using the Halosperm G2 test kit. Fertil Steril 2014;102:981-8. [Crossref] [PubMed]
- Ribeiro S, Sharma R, Gupta S, et al. Inter- and intra-laboratory standardization of TUNEL assay for assessment of sperm DNA fragmentation. Andrology 2017;5:477-85. [Crossref] [PubMed]
- Sharma RK, Sabanegh E, Mahfouz R, et al. TUNEL as a test for sperm DNA damage in the evaluation of male infertility. Urology 2010;76:1380-6. [Crossref] [PubMed]
- Fernández JL, Muriel L, Goyanes V, et al. Simple determination of human sperm DNA fragmentation with an improved sperm chromatin dispersion test. Fertil Steril 2005;84:833-42. [Crossref] [PubMed]
- Giwercman A, Richthoff J, Hjøllund H, et al. Correlation between sperm motility and sperm chromatin structure assay parameters. Fertil Steril 2003;80:1404-12. [Crossref] [PubMed]
- Giwercman A, Spano M, Lähdetie J, et al. Quality assurance of semen analysis in multicenter studies. Asclepios. Scand J Work Environ Health 1999;25 suppl 1:23-5. [PubMed]
- LeSaint C, Vingataramin L, Alix S, et al. Correlation between two sperm DNA fragmentation tests (TUNEL and SCSA) and evaluation of TUNEL assay inter-lab variabiality. Fertil Steril 2016;106:e297. [Crossref]
- Ribas-Maynou J, Garcia-Peiro A, Fernandez-Encinas A, et al. Comprehensive analysis of sperm DNA fragmentation by five different assays: TUNEL assay, SCSA, SCD test and alkaline and neutral Comet assay. Andrology 2013;1:715-22. [Crossref] [PubMed]
- Wiweko B, Utami P. Predictive value of sperm deoxyribonucleic acid (DNA) fragmentation index in male infertility. Basic Clin Androl 2017;27:1. [Crossref] [PubMed]
- López G, Lafuente R, Checa MA, et al. Diagnostic value of sperm DNA fragmentation and sperm high-magnification for predicting outcome of assisted reproduction treatment. Asian J Androl 2013;15:790-4. [Crossref] [PubMed]
- Bungum M, Humaidan P, Spano M, et al. The predictive value of sperm chromatin structure assay (SCSA) parameters for the outcome of intrauterine insemination, IVF and ICSI. Hum Reprod 2004;19:1401-8. [Crossref] [PubMed]
- Rilcheva VS, Ayvazova NP, Ilieva LO, et al. Sperm DNA Integrity Test and Assisted Reproductive Technology (Art) Outcome. J Biomed Clin Res 2016;9:21-9. [Crossref]
- Evenson DP. Sperm chromatin structure assay (SCSA®). Methods Mol Biol 2013;927:147-64. [Crossref] [PubMed]
- Evenson DP, Wixon R. Clinical aspect of sperm DNA fragmentation detection and male infertility. Theriogenology 2006;65:979-91. [Crossref] [PubMed]
- Meseguer M, Santiso R, Garrido N, et al. Effect of sperm DNA fragmentation on pregnancy outcome depends on oocyte quality. Fertil Steril 2011;95:124-8. [Crossref] [PubMed]
- Ahmadi A, Ng SC. Fertilizing ability of DNA-damaged spermatozoa. J Exp Zool 1999;284:696-704. [Crossref] [PubMed]
- García-Díaz M, Domínguez O, López-Fernández LA, et al. DNA polymerase lambda (Pol lambda), a novel eukaryotic DNA polymerase with a potential role in meiosis. J Mol Biol 2000;301:851-67. [Crossref] [PubMed]
- Allegrucci C, Thurston A, Lucas E, et al. Epigenetics and the germ line. Reproduction 2005;129:137-49. [Crossref] [PubMed]
- Dada R, Kumar M, Fernández JL, et al. Epigenetics and its role in male infertility. J Assist Reprod Genet 2012;29:213-23. [Crossref] [PubMed]
- Shapiro JA. A. 21st century view of evolution: genome system architecture, repetitive DNA, and natural genetic engineering. Gene 2005;345:91-100. [Crossref] [PubMed]
- Foster SS, De S, Johnson LK, et al. Cell cycle- and DNA repair pathway-specific effects of apoptosis on tumor suppression. Proc Natl Acad Sci U S A 2012;109:9953-8. [Crossref] [PubMed]
- Lips J, Kaina B. DNA double-strand breaks trigger apoptosis in p53-deficient fibroblasts. Carcinogenesis 2001;22:579-85. [Crossref] [PubMed]
- Niu ZH, Shi HJ, Zhang HQ, et al. Sperm chromatin structure assay results after swim-up are related only to embryo quality but not to fertilization and pregnancy rates following IVF. Asian J Androl 2011;13:862-6. [Crossref] [PubMed]
- Zini A, Finelli A, Phang D, et al. Influence of semen processing technique on human sperm DNA integrity. Urology 2000;56:1081-4. [Crossref] [PubMed]
- Zini A, Nam RK, Mak V, et al. Influence of initial semen quality on the integrity of human sperm DNA following semen processing. Fertil Steril 2000;74:824-7. [Crossref] [PubMed]
- Wdowiak A, Bojar I. Relationship between pregnancy, embryo development, and sperm deoxyribonucleic acid fragmentation dynamics. Saudi J Biol Sci 2016;23:598-606. [Crossref] [PubMed]
- Bach PV, Schlegel PN. Sperm DNA damage and its role in IVF and ICSI. Basic Clin Androl 2016;26:15. [Crossref] [PubMed]
- Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile male: a committee opinion. Fertil Steril 2015;103:e18-25. [Crossref] [PubMed]
- Jarrow J, Sigman M, Kolettis PN, et al. The optimal evaluation of the infertile male: best practice statement reviewed and validity confirmed 2011. Available online: https://www.auanet.org/education/guidelines/male-infertility-d.cfm
- Jungwirth A, Diemer T, Dohle GR, et al. Guidelines on male infertility. Available online: https://uroweb.org/guideline/male-infertility/
- Ni K, Steger K, Yang H, et al. Sperm protamine mRNA ratio and DNA fragmentation index represent reliable clinical biomarkers for men with varicocele after microsurgical varicocele ligation. J Urol 2014;192:170-6. [Crossref] [PubMed]
- Krishna Reddy SV, Shaik AB, Sailaja S, et al. Outcome of varicocelectomy with different degrees of clinical varicocele in infertile men. Advances in Andrology 2015;9.
- Vandekerckhove FW, De Croo I, Gerris J, et al. Sperm chromatin dispersion test before sperm preparation is predictive of clinical pregnancy in cases of unexplained infertility treated with intrauterine insemination and induction with clomiphene citrate. Front Med (Lausanne) 2016;3:63. [Crossref] [PubMed]
- Bareh GM, Jacoby E, Binkley P, et al. Sperm deoxyribonucleic acid fragmentation assessment in normozoospermic male partners of couples with unexplained recurrent pregnancy loss: a prospective study. Fertil Steril 2016;105:329-36.e1. [Crossref] [PubMed]
- Zidi-Jrah I, Hajlaoui A, Mougou-Zerelli S, et al. Relationship between sperm aneuploidy, sperm DNA integrity, chromatin packaging, traditional semen parameters, and recurrent pregnancy loss. Fertil Steril 2016;105:58-64. [Crossref] [PubMed]
- Carlini T, Paoli D, Pelloni M, et al. Sperm DNA fragmentation in Italian couples with recur-rent pregnancy loss. Reprod Biomed Online 2017;34:58-65. [Crossref] [PubMed]
- Osman A, Alsomait H, Seshadri S, et al. The effect of sperm DNA fragmentation on live birth rate after IVF or ICSI: a systematic review and meta-analysis. Reprod Biomed Online 2015;30:120-7. [Crossref] [PubMed]
- Jin J, Pan C, Fei Q, et al. Effect of sperm DNA fragmentation on the clinical outcomes for in vitro fertilization and intracytoplasmic sperm injection in women with different ovarian reserves. Fertil Steril 2015;103:910-6. [Crossref] [PubMed]
- Esteves SC, Sánchez-Martín F, Sánchez-Martín P, et al. Comparison of reproductive outcome in oligozoospermic men with high sperm DNA fragmentation undergoing intracytoplasmic sperm injection with ejaculated and testicular sperm. Fertil Steril 2015;104:1398-405. [Crossref] [PubMed]
- Pabuccu EG, Caglar GS, Tangal S, et al. Testicular versus ejaculated spermatozoa in ICSI cycles of normozoospermic men with high sperm DNA fragmentation and previous ART failures. Andrologia 2017;49. [Crossref] [PubMed]
- Bradley CK, McArthur SJ, Gee AJ, et al. Intervention improves assisted conception intracytoplasmic sperm injection outcomes for patients with high levels of sperm DNA fragmentation: a retrospective analysis. Andrology 2016;4:903-10. [Crossref] [PubMed]
- Arafa MM, ElBardisi HT, AlSaid SS, et al. Outcome of microsurgical testicular sperm extraction in familial idiopathic nonobstructive azoospermia. Andrologia 2015;47:1062-7. [Crossref] [PubMed]
- Esteves SC, Roque M, Bradley CK, et al. Reproductive outcomes of testicular versus ejaculated sperm for intracytoplasmic sperm injection among men with high levels of DNA fragmentation in semen: systematic review and meta-analysis. Fertil Steril 2017;108:456-67. [Crossref] [PubMed]
- Goldhaber-Fiebert JD, Brandeau ML. Evaluating cost-effectiveness of interventions that affect fertility and childbearing: how health effects are measured matters. Med Decis Making 2015;35:818-46. [Crossref] [PubMed]
- Wang YJ, Zhang RQ, Lin YJ, et al. Relationship between varicocele and sperm DNA damage and the effect of varicocele repair: a meta-analysis. Reprod Biomed Online 2012;25:307-14. [Crossref] [PubMed]
- Tunc O, Thompson J, Tremellen K. Improvement in sperm DNA quality using an oral antioxidant therapy. Reprod Biomed Online 2009;18:761-8. [Crossref] [PubMed]
- Ménézo YJ, Hazout A, Panteix G, et al. Antioxidants to reduce sperm DNA fragmentation: an unexpected adverseeffect. Reprod Biomed Online 2007;14:418-21. [Crossref] [PubMed]
- Kodama H, Yamaguchi R, Fukuda J, et al. Increased oxidative deoxyribonucleic acid damage in the spermatozoa of infertile male patients. Fertil Steril 1997;68:519-24. [Crossref] [PubMed]
- Omu AE, Al-Azemi MK, Kehinde EO, et al. Indications of the mechanisms involved in improved sperm parameters by zinc therapy. Med Princ Pract 2008;17:108-16. [Crossref] [PubMed]
- Greco E, Romano S, Iacobelli M, et al. ICSI in cases of sperm DNA damage: beneficial effect of oral antioxidant treatment. Hum Reprod 2005;20:2590-4. [Crossref] [PubMed]
- Greco E, Iacobelli M, Rienzi L, et al. Reduction of the incidence of sperm DNA fragmentation by oral antioxidant treatment. J Androl 2005;26:349-53. [Crossref] [PubMed]
- Martínez-Soto JC, Domingo JC, Cordobilla B, et al. Dietary supplementation with docosahexaenoic acid (DHA) improves seminal antioxidant status and decreases sperm DNA fragmentation. Syst Biol Reprod Med 2016;62:387-95. [Crossref] [PubMed]
- Fraga CG, Motchnik PA, Shigenaga MK, et al. Ascorbic acid protects against endogenous oxidative DNA damage in human sperm. Proc Natl Acad Sci U S A 1991;88:11003-6. [Crossref] [PubMed]
- Abad C, Amengual MJ, Gosalvez J, et al. Effects of oral antioxidant treatment upon the dynamics of human sperm DNA fragmentation and subpopulations of sperm with highly degraded DNA. Andrologia 2013;45:211-6. [Crossref] [PubMed]
- Vani K, Kurakula M, Syed R, et al. Clinical relevance of vitamin C among lead-exposed infertile men. Genet Test Mol Biomarkers 2012;16:1001-6. [Crossref] [PubMed]
- Gual-Frau J, Abad C, Amengual MJ, et al. Oral antioxidant treatment partly improves integrity of human sperm DNA in infertile grade I varicocele patients. Hum Fertil (Camb) 2015;18:225-9. [Crossref] [PubMed]
- Piomboni P, Gambera L, Serafini F, et al. Sperm quality improvement after natural anti-oxidant treatment of asthenoteratospermic men with leukocytospermia. Asian J Androl 2008;10:201-6. [Crossref] [PubMed]
- Kumar SB, Chawla B, Bisht S, et al. Tobacco use increases oxidative DNA damage in sperm – possible etiology of childhood cancer. Asian Pac J Cancer Prev 2015;16:6967-72. [Crossref] [PubMed]
- Jurewicz J, Radwan M, Sobala W, et al. Dietary patterns and their relationship with semen quality. Am J Mens Health 2016. [Epub ahead of print].
- Rima D, Shiv BK, Bhavna CH, et al. Oxidative stress induced damage to paternal genome and impact of meditation and yoga - Can it reduce incidence of childhood cancer? Asian Pac J Cancer Prev 2016;17:4517-25. [PubMed]
- Gosálvez J, González-Martínez M, López-Fernández C, et al. Shorter abstinence decreases sperm deoxyribonucleic acid fragmentation in ejaculate. Fertil Steril 2011;96:1083-6. [Crossref] [PubMed]
- Agarwal A, Gupta S, Du Plessis S, et al. Abstinence time and its impact on basic and advanced semen parameters. Urology 2016;94:102-10. [Crossref] [PubMed]
- Gosálvez J, de la Torre J, López-Fernández C, et al. DNA fragmentation dynamics in fresh versus frozen thawed plus gradient-isolated human spermatozoa. Syst Biol Reprod Med 2010;56:27-36. [Crossref] [PubMed]
- Xue X, Wang WS, Shi JZ, et al. Efficacy of swim-up versus density gradient centrifugation in improving sperm deformity rate and DNA fragmentation index in semen samples from teratozoospermic patients. J Assist Reprod Genet 2014;31:1161-6. [Crossref] [PubMed]
- Parmegiani L, Cognigni GE, Bernardi S, et al. ‘Physiologic ICSI’’, Hyaluronic acid (HA) favors selection of spermatozoa without DNA fragmentation and with normal nucleus, resulting in improvement of embryo quality. Fertil Steril 2010;93:598-604. [Crossref] [PubMed]
- Lee TH, Liu CH, Shih YT, et al. Magnetic-activated cell sorting for sperm preparation reduces spermatozoa with apoptotic markers and improves the acrosome reaction in couples with unexplained infertility. Hum Reprod 2010;25:839-46. [Crossref] [PubMed]
- Nadalini M, Tarozzi N, Di Santo M, et al. Annexin V magnetic-activated cell sorting versus swim-up for the selection of human sperm in ART: is the new approach better then the traditional one? J Assist Reprod Genet 2014;31:1045-51. [Crossref] [PubMed]
- Rashki Ghaleno L, Rezazadeh Valojerdi M, Chehrazi M, et al. Hyaluronic acid binding assay is highly sensitive to select human spermatozoa with good progressive motility, morphology, and nuclear Maturity. Gynecol Obstet Invest 2016;81:244-50. [Crossref] [PubMed]
- Hammoud I, Boitrelle F, Ferfouri F, et al. Selection of normal spermatozoa with a vacuole-free head (x6300) improves selection of spermatozoa with intact DNA in patients with high sperm DNA fragmentation rates. Andrologia 2013;45:163-70. [Crossref] [PubMed]
- Maettner R, Sterzik K, Isachenko V, et al. Quality of human spermatozoa: relationship between highmagnification sperm morphology and DNA integrity. Andrologia 2014;46:547-55. [Crossref] [PubMed]
- Rappa KL, Rodriguez HF, Hakkarainen GC, et al. Sperm processing for advanced reproductive technologies: Where are we today? Biotechnol Adv 2016;34:578-87. [Crossref] [PubMed]
- Greco E, Scarselli F, Iacobelli M, et al. Efficient treatment of infertility due to sperm DNA dmage by ICSI with testicular spermatozoa. Hum Reprod 2005;20:226-30. [Crossref] [PubMed]
- Moskovtsev SI, Jarvi K, Mullen JB, et al. Testicular spermatozoa have statistically significantly lower DNA damage compared with ejaculated spermatozoa in patients with unsuccessful oral antioxidant treatment. Fertil Steril 2010;93:1142-6. [Crossref] [PubMed]
- Smith R, Kaune H, Parodi D, et al. Increased sperm DNA damage in patients with varicocele: relationship with seminal oxidative stress. Hum Reprod 2006;21:986-93. [Crossref] [PubMed]
- Werthman P, Wixon R, Kasperson K, et al. Significant decrease in sperm deoxyribonucleic acid fragmentation after varicocelectomy. Fertil Steril 2008;90:1800-4. [Crossref] [PubMed]
- Moskovtsev SI, Lecker I, Mullen JB, et al. Cause-specific treatment in patients with high sperm DNA damage resulted in significant DNA improvement. Syst Biol Reprod Med 2009;55:109-15. [Crossref] [PubMed]
- Agarwal A, Cho CL, Majzoub A, et al. Editorial: Frontiers in Clinical Andrology. Transl Androl Urol 2017;6:S343-5.
- Evgeni E, Charalabopoulos K, Asimakopoulos B. Human sperm DNA fragmentation and its correlation with conventional semen parameters. J Reprod Infertil 2014;15:2-14. [PubMed]
- Practice Committee of the American Society for Reproductive Medicine. Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril 2015;103:e9-17. [Crossref] [PubMed]
- Cissen M, Wely MV, Scholten I, et al. Measuring sperm DNA fragmentation and clinical outcomes of medically assisted reproduction: A systematic review and meta-analysis. PLoS One 2016;11:e0165125. [Crossref] [PubMed]
- Chenlo PH, Curi SM, Pugliese MN, et al. Fragmentation of sperm DNA using the TUNEL method. Actas Urol Esp 2014;38:608-12. [Crossref] [PubMed]
- Zini A. Are sperm chromatin and DNA defects relevant in the clinic? Syst Biol Reprod Med 2011;57:78-85. [Crossref] [PubMed]
- Lin MH, Kuo-Kuang Lee R, Li SH, et al. Sperm chromatin structure assay parameters are not related to fertilization rates, embryo quality, and pregnancy rates in in vitro fertilization and intracytoplasmic sperm injection, but might be related to spontaneous abortion rates. Fertil Steril 2008;90:352-9. [Crossref] [PubMed]
- Robinson L, Gallos ID, Conner SJ, et al. The effect of sperm DNA fragmentation on miscarriage rates: a systematic review and meta-analysis. Hum Reprod 2012;27:2908-17. [Crossref] [PubMed]
- Simon L, Murphy K, Shamsi MB, et al. Paternal influence of sperm DNA integrity on early embryonic development. Hum Reprod 2014;29:2402-12. [Crossref] [PubMed]
- Tesarik J, Greco E, Mendoza C. Late, but not early, paternal effect on human embryo development is related to sperm DNA fragmentation. Hum Reprod 2004;19:611-5. [Crossref] [PubMed]
- Shridharani A, Owen RC, Elkelany OO, Kim ED. The significance of clinical practice guidelines on adult varicocele detection and management. Asian J Androl 2016;18:269-75. [Crossref] [PubMed]
- Society for Translational Medicine. Available online: http://www.thestm.org/
- Agarwal A, Cho CL, Majzoub A, et al. The Society for Translational Medicine: clinical practice guidelines for sperm DNA fragmentation testing in male infertility. Transl Androl Urol 2017;6:S720-33.
- Cho CL, Agarwal A, Majzoub A, et al. Clinical utility of sperm DNA fragmentation testing: concise practice recommendations. Transl Androl Urol 2017;6:S366-73.
- Da Aw L, Zain MM, Esteves SC, et al. Persistent Mullerian Duct Syndrome: a rare entity with a rare presentation in need of multidisciplinary management. Int Braz J Urol 2016;42:1237-43. [Crossref] [PubMed]
- Povlsen BB, Aw LD, Laursen RJ, et al. Pregnancy and birth after intracytoplasmic sperm injection with normal testicular spermatozoa in a patient with azoospermia and tail stump epididymal sperm. Int Braz J Urol 2015;41:1220-5. [Crossref] [PubMed]