
Neonatal network database operated by the Neonatal Research Network of Japan
Background
The database for high-risk infants (HRIs) in Japan was first established in 2003 by the research group (principal investigator: Masanori Fujimura) of the Japanese Ministry of Health, Labour and Welfare Science Research Project, titled “Perinatal Center Network” (1). At that time, the British Association of Perinatal Medicine (BAPM) database was already established in the United Kingdom (2), and the National Institute of Child Health and Development (NICHD) and Vermont-Oxford Network (VON) network databases were also established in the USA (3,4). Finally, the Japanese database for HRIs was launched more than 10 years later than those in these countries. The basic database structure was discussed and decided by this research group, and actual data registration began with infants born in 2003. From the beginning, registration has been based on each participant’s hospital. Therefore, there is no cost for registration or compensation for participants. Because the subjects for registration were decided based on pre-existing databases such as BAPM, NICHD, and VON, infants born at or less than 1,500 g were included at the beginning, and then infants born before 32 weeks of gestation have also been included since 2015. The number of variables for facilities was 14 and for infants, 81. The items for registration were also decided by referring to pre-existing databases. Furthermore, the items’ definitions were matched with these databases as much as possible so that international comparisons could be made. The current database consists of 155 variables for infants (table online only: https://cdn.amegroups.cn/static/public/pm-21-71-01.xlsx). All variables were collected until neonatal intensive care unit (NICU) discharge. In addition to variables in the NICU, the Japanese database has also been collecting follow-up data at 1.5, 3, and 6 years of age. This was a unique feature of the Japanese database compared to other existing databases. Follow-up data includes 58 variables at 1.5 years old, 147 at 3 years old, and 193 at 6 years old. From the beginning, there has been a firm belief that the outcomes of HRIs should include not only morbidities during NICU stay but also neurological development, including visual and hearing ability and physical growth after discharge at the ages of 1.5 and 3 years old. For the first two years, the data were registered by sending an Excel file to the secretariat, but from 2005, all information has been registered on the specific website.
Organization and structure
Organization
The network database project was supported by a grant from the Japanese Ministry of Health, Labour and Welfare for eight years from 2004 to 2011. However, for two years from 2012, it was operated with research funds from the Tokyo Women’s Medical University Maternal and Perinatal Center without any grants. Then, in 2014, the nonprofit organization (NPO) the Neonatal Research Network of Japan (NRNJ) was established with support from academic societies such as the Japanese Society of Neonatal Medicine and Development and the Japanese Society for Perinatal and Neonatal Medicine. Currently, the NRNJ is operated independently by donations. The network has six directors, two auditors, and three part-time clerks and has an office in Shinjuku, Tokyo to operate the database (5).
Participating NICUs
Participation in this network database is voluntarily decided. There are about 100 Level III and about 300 Level II perinatal centers in Japan. Only 40 perinatal centers participated when the database started in 2003. Since then, the number of participating hospitals has increased, and currently, about 170 hospitals are constantly participating (6). However, about half of all participating hospitals are Level III perinatal centers, indicating that relatively large-scale Level III perinatal centers are the predominant participants among all perinatal centers. Therefore, the database coverage rate is higher than the participation rate, and it has remained at about 65% of the total population of HRIs born in Japan.
Objectives
In order to improve the prognosis of HRIs especially those born with a very low birth weight (VLBW) or with a gestational age less than 32 weeks [very preterm (VP)], it was necessary to know the current outcomes as the first step. Therefore, it was essential to establish a database that consists of multiple perinatal centers and that accumulates data over time among HRIs in Japan. Otherwise, the necessary steps for improving outcomes could not be discussed. Therefore, the first objective of the database is to evaluate the actual prognosis of HRIs.
Next, once the actual outcomes situation became clear, the measures to be taken for improving outcomes also became apparent. In particular, the first analysis after the database was established showed that there was a large center variation in outcomes among the participating perinatal centers (7). Moreover, the difference among the centers was more than expected. This existence of inter-center variation in outcomes suggested that there was room for improving the outcomes in the entire network by improving the prognosis of poorly performing perinatal centers. Therefore, the next purpose of the network database is to use the database in order to improve the outcomes in the entire network. Specifically, in order to achieve this, the following activities have been applied: benchmarking, quality improving (QI) conference, learning from high performing centers, and interventional research.
The third purpose is to use the aggregated data to explain the updated status of HRI in outcomes in Japan to HRIs’ families. Of course, the mortality outcome for HRI is 0 or 100%, which cannot be expressed using percentage. However, we believe that the accumulated data of a large number of infants could serve as an overview for the involved families to some extent.
Finally, by proactively disclosing the prognosis of HRIs in Japan at academic societies, etc., an understanding of HRI medical care can be promoted by all stakeholders. Although the outcomes of individual centers are disclosed anonymously, each center recognizes its ranking. This feedback can serve as an impetus for administrative persons in a given center to promote the perinatal medical system. In addition, this improvement must be activated on a prefectural basis since perinatal systems are operated and maintained by local governments. This is useful for the advocacy of perinatal medicine.
Missions
Improving the outcomes of HRIs in Japan is the database’s primary mission, but other core goals include information sharing through QI activities, the standardization of neonatal intensive care for HRI, and publishing manuals for standardized neonatal care. Furthermore, in order to provide opportunities for epidemiological research using databases, educational guidance and training for young researchers provide by statistics experts is also included in the mission.
Visions
Since participation in the network is carried out on a volunteer basis, the benefits of joining this database project should be further enhanced and increasing the number of participating centers should also be a goal. For this purpose, further strengthening the QI activities is essential. On the other hand, the largest barrier to participating in this database project is the manpower burden of data entry. Therefore, a system that enables the automatic input of data from an electronic medical record (EMR) is needed. Currently, some centers possess the ability to extract necessary information from medical records, however, the development of a universal system needs to be accelerated.
Activities
Data registry
All necessary data can be registered through a designated website. It is encouraged that all items during an infant’s NICU stay are to be uploaded as soon as they are discharged from the NICU. Then, when medical follow-up examinations are performed at the ages of 1.5, 3, and 6 years, follow-up information can be registered. Each participating center can log in to the site with their unique identification number (ID) and password (PW) and register their data. Because all registered information is stored as numerical and text data with a center-specific ID, even if a third party happens to download the data, it is not possible to decrypt the data content without knowing the data structure. Furthermore, even if the data structure is clarified somehow, it would not be possible to identify any individuals since identifying information such as dates of birth is not originally registered and hospital IDs are not opened.
Analysis and feedback of registered data
The registered data are aggregated, and feedback is given to the participating centers once a year. The feedback includes summarized results of all participating centers and the data unique to each center. Therefore, each center can compare its outcomes with the average of the whole network (Figure 1). Furthermore, each center can learn its rank as a benchmark. Figure 2 shows the frequency of cord blood transfusions and each center’s ranking with regard to these. Any given center can determine the frequency of their own facility’s transfusion rate using the center number provided in their feedback. However, it is not possible to determine the center number of others. Similarly, Figure 3 shows the mortality rate ranking. However, the mortality rate may vary in the case mix. Therefore, the feedback also contains the adjusted mortality rate obtained with a 95% confidence limit (Figure 4). In addition, a radar chart, as shown in Figure 5 is also provided.
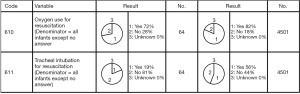
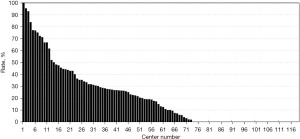
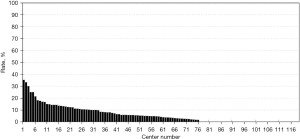
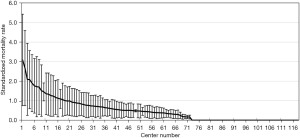
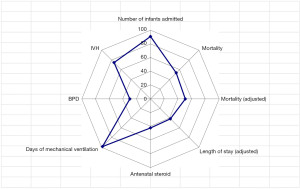
Until 2016, these feedback data were printed and distributed to participating centers as an annual report, but currently, all feedback data can be downloaded as PDF files from the website. The entirely aggregated data are also published on the website (6).
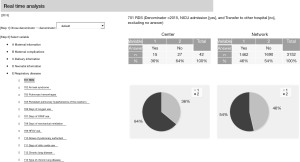
Furthermore, a function to perform real-time analysis for comparison is now available on the website (Figure 6). Using this function, all participating centers can compare their outcomes with the entire network at any time, even during the NICU round.
Quality improvement conference
Once a year, a QI conference is held by gathering representatives from participating centers. The first QI conference was held in 2007. The content of these conferences involves discussing strategies to reduce center variation, trends in mortality and morbidities, the prevention of major morbidities, the improvement of database items, etc., in addition to annual database analysis reports. Well-performing centers, especially with regards to the survival rate among infants born at less than 24 weeks of gestation or the rate of chronic lung disease, have introduced their daily management procedures at these conferences as a standard of treatment.
Since these conferences are the only opportunity for representatives of participating centers to gather, we also discuss several issues regarding the operation of the network database. The addition or modification of registered variables also must be discussed and decided at these conferences.
Ten-year anniversary project
Because the Japanese database started in 2003, a specific analysis project targeting the database’s first 10 years began in 2015. During the 10 years, from 2003 to 2012, a total of 40,806 infants were registered in the database. The project started with asking all participating centers if they would be interested in joining the project. Some 20 centers expressed their intention to involve the project, and these were then asked about their theme of analysis. After adjusting the theme for each center, the person in charge of each theme was decided. Each investigator received the dataset and stated analysis on the predetermined objective. The analysis results were submitted to several international journals, and eventually, more than 20 manuscripts were published (8).
Besides individual analysis, a comprehensive analysis of the dataset was also performed, and the analyzed results were published as printed material and a PDF file on the website (9). This comprehensive analysis was followed by the addition issues, volumes 1 and 2 (10,11).
Publication of NICU manual
As the number of published manuscripts on the database increased, many questions regarding procedures and treatment in Japanese NICUs were received from outside of Japan. Therefore, a manual titled “Neonatal Intensive Care Manual for the infants born less than 28 weeks of gestation” was published and posted on the website in 2019 (12). Once the manual was published, it became clear that there were similarities and differences globally in the procedures and treatments used for extremely preterm infants. Sharing and exchanging information within a country and among countries is another approach that can be used to improve the outcomes of HRIs.
Lectures by biostatisticians
At QI meetings, we have held lectures by biostatistics experts in order to disseminate knowledge about the analysis of registered databases. The data themselves contain information on many infants. Therefore, each investigator that analyzes the big data requires a certain level of knowledge on the statistical methods used in the database. To acquire this knowledge, several lectures by biostatisticians have been held, which have been supported by the network.
Web-based lectures
Because it has not been possible to host face-to-face lectures recently due to the COVID-19 pandemic, web-based lectures are currently scheduled once a month. Speakers at these lectures have included the first authors of published manuscripts analyzing the network database. During these lectures, as well as introducing their published manuscripts, the authors have explained tips and difficulties for data analysis and publishing. Therefore, the lectures were and will be valuable for young investigators.
Support of clinical research
In addition to operating the database, several grants for neonatal clinical research have been provided to investigators. Eligibility and permission for grants must be approved by a committee consisting of the board members of the NRNJ.
Current within the country and outside of country collaborations
Joint research in Japan
A project linking this neonatal database with other databases such as those involving maternal data and reimbursement information is now under consideration.
International joint research
Providing data to VON
A limited number of centers in Japan used to provide their data to the VON, but currently, no center provides their data to them due to the cost of participation.
Participation in the International Network for Evaluating Outcomes (iNeo)
From the beginning of the iNeo, the Japanese data have been provided to (13). International comparisons of data are extremely useful (14). This international collaborative research is reported in detail separately in this journal, so an explanation in this section is omitted.
Participation in the International Neonatal Consortium (INC)
The type of data necessary to develop drugs for neonates is discussed by the INC (15). In addition, the network data will be used for a Real World Data (RWD) project in the INC.
Joint research with the California Perinatal Quality Care Collaborative (CPQCC)
A part of the Japanese network data was shared with the CPQCC, which is a perinatal network in California, USA. Because California and Japan are relatively similar in area and population size, a comparison of the two network databases is mutually beneficial. As a result of this joint research, we recently examined the outcomes of VLBW infants born to Japanese mothers in California and those of infants born in Japan (16). This kind of study can provide a new perspective for future joint projects among network databases.
Engagement activities for participating members
Individual research
All participating centers have the right to use the network database for their own research purposes. All accumulated data can be provided to a primary investigator from each center with an official application. The analysis subject and theme are entirely up to the applicant. However, the theme of analysis, including the purpose and subjects, should be provided when applying for data. If a planned analysis theme has a similarity to previous research, the applicant could be asked to change their theme. The data provided are not transferable to a third party and should be deleted 3 years after the application.
Clinical trials
Cluster randomized controlled trial
A cluster randomized controlled trial was conducted to show the benefit of Bifidobacterium administration to VLBW infants by dividing the participating centers into an intervention group with Bifidobacterium and a control group, that is, by using a cluster randomization method (17). The centers that participated in this study did not use a special case registration form but simply registered the data in the network database with the usual medical care. Centers in the treatment group were asked to provide Bifidobacterium to all VLBW infants as if it were a routine procedure. By using the existing network database, this study serves as an example for future clinical trials.
Interventional trial
Using the data registered in the network database, the medical care strengths and weaknesses of each participating center were calculated. Then an interventional study was conducted to determine the effectiveness of interventions into weaknesses of the centers. This study was named the INTACT (Improvement of NICU practice and Team Approach Cluster randomized controlled Trial) study. The interventions included holding workshops for the improvement of weaknesses and external outreach education. The outcome of hospitalized VLBW infants was a primary outcome. Currently, the results are being analyzed (18).
Funding support
The operation cost for the network database is currently covered by donations from individuals, academic societies, and companies. There is no special cost for participating centers. The average running cost of the network is around 50,000 USD per year.
Challenges
At the beginning of the database, there were 81 variables regarding NICU stay and 17 each follow-up data for 1.5 and 3 years of age, but the number of variables has gradually increased. Currently, as mentioned before, there are 155 items registered for NICU stay and 58 and 147 items for outcomes at 1.5 and 3 years of age, respectively. In addition, from 2020 onward, 193 variables for outcomes at 6 years of age have been added. Subsequently, the burden on data entry persons is increasing. Therefore, it is necessary to introduce an automatic extraction system from EMRs in the future.
Future directions
RWD project
Some of the network data will be provided to the RWD project conducted by the INC. It is expected that these data will lead to the promotion of the development of neonatal drug therapy.
Improvement of coverage rate
We aim to raise the coverage rate by disseminating the advantages of participating in the network even to relatively small NICUs. On the other hand, if only increasing the coverage rate is pursued, it may be difficult to guarantee the quality of the data, so it is important to strike the balance between the quality of the data and the coverage rate.
Although the Japanese database includes long-term outcome data after NICU discharge, there is no systematic follow up system for infants after discharge, unlike clinical trials, and each center has been conducting its follow-ups for HRIs on a voluntary basis, that is, depending on parents’ wishes. Therefore, the overall follow-up rate is about 60%. However, conversely, it is surprising that about 60% of HRIs discharged from NICUs are followed up at the same perinatal centers even after discharge, not for research purposes. In the future, even if infants and their families move after discharge, a system should be built in collaboration with the Japan Neonatal Follow-up Study Group so that the follow-up data can be collected across the whole network. This study group was founded in 1994. The members were clinical follow-up staff for VLBW infants. The majority of them were neonatologists and psychologists. The group has expanded in size since the initiation of the Japanese network database in 2003. Their significant contributions to the network database have included establishing a follow-up system, publishing a manual for follow-up and disseminating the psychological evaluation skills of preterm infants who are subjects in the NRNJ.
Conclusions
Since the inception of this Japanese network database, approximately 15 years have passed. We believe that the current status and future possibilities of the database have attracted and will continue to attract worldwide recognition and esteem. Further international collaborative research will proceed through this database in the future.
Acknowledgments
The authors would like to thank all of the participating hospitals and infants involved in the study for their valuable contributions. List of participating NICUs: Sapporo City Hospital, Asahikawa Kosei Hospital, Kushiro Red Cross Hospital, Obihiro Kosei Hospital, Tenshi Hospital, JCHO Hokkaido Hospital, NTT East Sappro Hospital, Nikko Kinen Hospital, Sapporo Prefecture Medical University, Asahikawa Medical University, Aomori Prefecture Central Hospital, Iwate Medical University, Iwate Prefecture Ninohe Hospital, Sendai Red Cross Hospital, Tohoku University, Akita Red Cross Hospital, Akita University, Tsuruoka City Shonai Hospital, Yamagata Prefecture Central Hospital, Fukusima Prefecture Medical University, National Fukushima Hospital, Tsukuba University, Ibaraki Children’s Hospital, Jichi Medical University, Ashikaga Red Cross Hospital, Gunma Prefecture Children’s Hospital, Kiryu Kosei General Hospital, Ohta General Hospital, Gunma University, Saitama Medical University, Saitama Prefecture Children’s Hospital, Saitama Medical University Medical Center, Kawaguchi City Medical Center, Chiba City Kaihin Hospital, Kameda General Hospital, Juntendo University Urayasu Hospital, Narita Red Cross Hospital, Tokyo Metropolitan Children’s Medical Center, Tokyo Women’s Medical University, Aiiku Hospital, Nihon University, Tokyo Medical University, Teikyo University, Showa University, Japan Red Cross Hospital, National Center for Child Health and Development, Tokyo University, Toho University, Tokyo Metropolitan Bokuto Hospital, Tokyo Medical and Dental University, Juntendo University, Katsushika Red Cross Hospital, Yokohama City University Medical Center, Marianna Medical University, Kanagawa Children’s Medical Center, Tokai University, Kitazato University, Yokosuka Kyosai Hospital, Nippon Medical School Musashi Kosugi Hospital, Yokohama City Hospital, Saiseikai Eastern Yokohama Hospital, Yokohama Medical Center, Yamanashi Prefecture Central Hospital, Nagano Children’s Hospital, Shinshu University, Nigata University, Niigata City Hospital, Nagaoka Red Cross Hospital, Toyama Prefectural Central Hospital, Toyama University, Ishikawa Prefectural Central Hospital, Gifu Prefectural Medical Center, Oogaki City Hospital, Seirei Hamamatsu Hospital, Shizuoka Saiseikai Hospital, Shizuoka Children’s Hospital, Hamamatsu Medical University, Nagoya Red Cross Daini Hospital, Nagoya University, Nagoya Red Cross Daiici Hospital, Toyohashi City Hospital, Fujita Medical University, Anjokosei Hospital, Koritsu Tosei Hospital, Toyota Memorial Hospital, Konankosei Hospital, Nogoya Chity University, Aichi Medical University, National Mie Central Medical Center, Otsu Red Cross Hospital, Shiga Medical University, Nagahama Red Cross Hospital, Japan Baptist Hospital, Kyoto University, Kyoto Red Cross Daiichi Hospital, National Maizuru Medical Center, Yodogawa Christian Hospital, Osaka Women’s and Children’s Hospital, Takatuski General Hospital, Kansai Medical University, Osaka City General Hospital, Saiseikai Suita Hospital, Bell Land General Hospital, Hannan Central Hospital, Osaka City University, Kobe Children’s Hospital, Kobe University, Saiseikai Hyogo Hospital, Kobe City Medical Center Central Hospital, Hyogo Medical University, Himeji Red Cross Hospital, Toyooka General Hospital, Nara Prefecture Medical University, Wakayama Prefecture Medical University, Tottori Prefectural Central Hospital, Matue Red Cross Hospital, Kurashiki Central Hospital, Kawasaki Medical University, National Okayama Medical Center, Hiroshima City Central Hospital, Hiroshima Prefectural Hospital, Yamaguchi University, Yamaguchi Prefecture Medical Center, Tokushima University, Tokushima Prefecture Central Hospital, Shikoku Medical Center for Children and Adults, Ehime Prefectural Cntral Hospital, Kochi Health Science Center, National Kyushu Medical Center, Kurume University, Kitakyushu City Hospital, University of Occupational and Environmental Health Japan, Fukuoka University, Kyushu University, Iizuka Hospital, National Kokura Medical Center, Fukuoka City Children’s Hospital, National Saga Hospital, National Nagasaki Medical Center, Saseho City Hospital, Kumamoto City Hospital, Kumamoto University, Oita Prefectural Hospital, Almeida Memorial Hospital, Miyazaki University, Kagosima City Hospital, Imakyure General Hospital, Okinawa Prefectural Nanbu Medical Center/Nanbu Child Medical Center, Okinara Prefectural Central Hospital, Okinawa Red Cross Hospital (as of April 1, 2021).
Funding: The network is operated by the nonprofitable organization Neonatal Research Network of Japan.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Shoo Lee and Prakesh Shah) for the series “Neonatal Networks for Outcomes Improvement: Evolution, Progress and Future” published in Pediatric Medicine. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://pm.amegroups.com/article/view/10.21037/pm-21-71/coif). The series “Neonatal Networks for Outcomes Improvement: Evolution, Progress and Future” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics board of Osaka Women’s and Children’s Hospital (No. 1104-4), and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ministry of Health and Labour, Japan [Internet]. Tokyo: Government [cited 2021 Oct 27]. Available online: https://mhlw-grants.niph.go.jp/project/9582/1 (Japanese).
- British Association of Perinatal Medicine [Internet]. London: Association [cited 2021 Oct 27]. Available online: https://www.bapm.org/pages/19-neonatal-networks/
- Neonatal Research Network, NICHD [Internet]. Washington: Government [cited 2021 Oct 27]. Available online: https://neonatal.rti.org/index.cfm?fuseaction=DataRequest.Home/
- Vermont Oxford Network [Internet]. Vermont: Organization. [cited 2021 Oct 27]. Available online: https://public.vtoxford.org/data-and-reports/
- Neonatal Research Network of Japan [Internet]. Tokyo: Organization [cited 2021 Oct 27]. Available online: http://nponrn.umin.jp/nrndata/indexe.htm/
- Neonatal Research Network of Japan [Internet]. Tokyo: Organization [cited 2021 Oct 27]. Available online: http://plaza.umin.ac.jp/nrndata/syukeie/
- Kusuda S, Fujimura M, Sakuma I, et al. Morbidity and mortality of infants with very low birth weight in Japan: center variation. Pediatrics 2006;118:e1130-8. [Crossref] [PubMed]
- Neonatal Research Network of Japan [Internet]. Tokyo: Organization [cited 2021 Oct 27]. Available online: http://plaza.umin.ac.jp/nrndata/pdf/publicationlist20200925.pdf/
- Neonatal Research Network of Japan [Internet]. Tokyo: Organization [cited 2021 Oct 27]. Available online: http://plaza.umin.ac.jp/nrndata/pdf/
- YearReport.pdf/10. Neonatal Research Network of Japan [Internet]. Tokyo: Organization [cited 2021 Oct 27]. Available online: http://plaza.umin.ac.jp/nrndata/pdf/10YearReportad.pdf/
- Neonatal Research Network of Japan [Internet]. Tokyo: Organization [cited 2021 Oct 27]. Available online: http://plaza.umin.ac.jp/nrndata/pdf/10YearReportad2.pdf/
- Neonatal Research Network of Japan [Internet]. Tokyo: Organization [cited 2021 Oct 27]. Available online: http://plaza.umin.ac.jp/nrndata/pdf/NICUManual.pdf/
- Shah PS, Lui K, Reichman B, et al. The International Network for Evaluating Outcomes (iNeo) of neonates: evolution, progress and opportunities. Transl Pediatr 2019;8:170-81. [Crossref] [PubMed]
- Shah PS, Lui K, Sjörs G, et al. Neonatal Outcomes of Very Low Birth Weight and Very Preterm Neonates: An International Comparison. J Pediatr 2016;177:144-152.e6. [Crossref] [PubMed]
- Costeloe K, Turner MA, Padula MA, et al. Sharing Data to Accelerate Medicine Development and Improve Neonatal Care: Data Standards and Harmonized Definitions. J Pediatr 2018;203:437-441.e1. [Crossref] [PubMed]
- Kusuda S, Bennett M, Gould J, et al. Outcomes of Infants with Very Low Birth Weight Associated with Birthplace Difference: A Retrospective Cohort Study of Births in Japan and California. J Pediatr 2021;229:182-190.e6. [Crossref] [PubMed]
- Totsu S, Yamasaki C, Terahara M, et al. Bifidobacterium and enteral feeding in preterm infants: cluster-randomized trial. Pediatr Int 2014;56:714-9. [Crossref] [PubMed]
- Improvement of NICU Practice and Team Approach Cluster Randomized Clinical Trial. [Internet] Tokyo: Organization. [cited 2021 Oct 27]. Available online: https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr_view.cgi?recptno=R000007185/
Cite this article as: Kusuda S, Fujimura M. Neonatal network database operated by the Neonatal Research Network of Japan. Pediatr Med 2023;6:19.

