
The influence of adjuvant radiation therapy after endoscopic resection on survival for early stage EC: an analysis of the surveillance epidemiology and end results (SEER) database
Introduction
Esophageal cancer (EC) incidence rates are rising each year, but treatment outcomes are still very poor (1). Due to increased awareness and progress in surveillance, many EC cases are being diagnosed in early stage. However, early stage EC is highly heterogeneous and has low representativeness, so very few phase III trials have investigated this population. Several treatment options are available for these patients including esophagectomy, radiation therapy (RT), chemotherapy, and endoscopic resection (ER) (2,3), but the optimum treatment strategies still remain unclear (4).
ER is less invasive than esophagectomy and it can radically resect the tumor while maintaining the integrity of the esophagus (5), but it cannot target the lymph nodes possibly involved in metastasis. Retrospective studies have shown that survival is comparable between ER and esophagectomy for early stage EC (6). Therefore, due to its distinct advantages, ER has been increasingly used for early stage EC treatment (7,8). It has been established as the standard treatment for EC clinically staged as T1a confined to the mucosa with low risk of lymph node metastasis (9). T1b EC is defined as submucosal cancer which has relatively high risk of metastasis (5). The risk of lymph node metastasis of T1b EC is mainly based on esophagectomy and secondarily based on definitive chemoradiotherapy (CRT) (10). However, as surgery is associated with increased morbidity and mortality, and definitive CRT often leads to adverse events, patients at high risk for lymph node metastasis may be unfit for surgery and CRT and are often managed conservatively (10). Studies have demonstrated that in the T1b EC patients ER was associated with lower rate of lymph node metastasis when compared with surgery (11-13). Thus, ER is a potential treatment option for T1b EC. T2 tumors remain in a middle ground between T1 tumors and the more advanced tumors of T3 or N1. The optimal treatment for N2 tumors is not clear and recommendations vary from surgery alone to multimodal treatment (4). Previous studies have indicated that curative treatment by ER can be achieved for over 33% of T2 EC patients (14).
ER followed by RT or CRT is a common combination treatment method for early stage ECs. It is effective for patients with early stage EC and has better or comparable efficacy with that of surgery or definitive CRT, as indicated by some retrospective studies (10,15-20). Addition of CRT aims to prevent lymph node recurrence, but there are very few studies validating the necessity of adjuvant CRT in this setting. Therefore, we performed this study based on the SEER database to understand the potential role of adjuvant RT after ER in the treatment of early stage EC patients.
Methods
Study population
The surveillance, epidemiology, and end results (SEER) database (National Cancer Institute) was queried with SEER*Stat 8.1.2 software for EC cases. The years of diagnosis were set to 1998–2013. The screening parameters were set as primary tumor site (esophageal), adults (age ≥18 years) and survival time. The following information was extracted: age, gender, race, histological types, tumor location, tumor stage and grade, ER, chemotherapy and RT, survival status and survival time. The 8th Edition of the American Joint Committee on Cancer (AJCC)/Union for International Cancer Control tumor-node-metastasis (TNM) staging system was used to redefine the clinical staging of tumors. The T1-2 stage EC cases in which ER was followed by radiation or observation were included in the present analysis.
Statistical analysis
SPSS Statistics 20.0 software (IBM Corp., Armonk, NY, USA) was used for statistical analysis. Continuous variables were presented as means ± standard deviation (SD). Category variables were presented as frequency. The clinical parameters between the patients who received radiation and those who did not were compared using t-tests for continuous variables and chi-square tests for categorical variables. Kaplan-Meier methods were performed to compare overall survival (OS) and cancer-specific survival (CSS) between the patients who received radiation and those who did not. Subgroup analysis was done with AJCC stage. Furthermore, we used a multivariate Cox proportional-hazards regression model to identify independent covariates that may influence survival. All tests were two-sided. A P value of less than 0.05 was considered statistically significant.
Results
Patient characteristics
A total of 759 early stage EC patients who received ER as primary treatment were included in this study, with 671 patients in the no-radiation group and 88 patients in the radiation group. In this patient population, 139 patients had EC clinically staged at T1, 473 patients had cT1a EC, 102 patients had cT1b EC, and 45 had a T2 staging. Adenocarcinomas were more common than squamous cell carcinomas (557 vs. 93 patients), which correlated with tumors located predominantly in the lower third of the esophagus (520 patients). The clinical characteristics of the patients included are shown in Table 1. Age and gender were not significantly different between the radiation group and no-radiation group (all P>0.05). However, there was a significant difference between the two groups when considering tumor location, pT stage, histologic types, race, tumor grade and chemotherapy (all P<0.05).
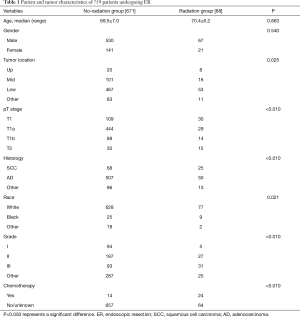
Full table
Relationship between RT after ER and OS or CSS outcomes in patients with cT1-2 EC
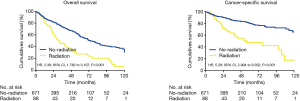
As shown in Figure 1, the median survival of the no-radiation group was significantly longer than that of the radiation group [74 vs. 31 months; hazard risk (HR), 2.39; 95% confidence interval (CI), 1.782–3.197; P<0.001]. Similarly, the CSS between the two groups also differed significantly, with a median survival of 97 months for the no-radiation group versus 64 months for the radiation group (HR, 5.29; 95% CI, 2.994–9.352; P<0.001) (Figure 1).
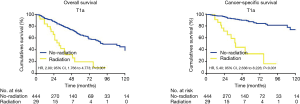
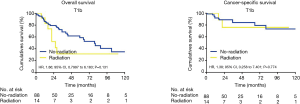
Due to the high heterogeneities between different stages of early EC, further subgroup analyses were made within the clinical stages of T1a, T1b and T2. In the T1a subgroup analysis, the patients who did not receive radiation had better OS and CSS when compared with the patients receiving RT (OS: 90 vs. 31 months; HR, 2.90; 95% CI, 1.766–4.773; P<0.001; CSS: 105 vs. 48 months; HR, 5.4; 95% CI, 2.636–8.226; P<0.001) (Figure 2). However, for the T1b subgroup, OS and CSS were not significantly different between the radiation group and the no-radiation group (OS: 21 vs. 97 months; HR, 1.86; 95% CI, 0.7997–6.183; P=0.131; CSS: 89 vs. 99 months; HR, 1.38; 95% CI, 0.258–7.401; P=0.774) (Figure 3). In the T2 subgroup analysis, the OS and CSS between the two groups also did not differ significantly (OS: 48 vs. 40 months; HR, 1.002; 95% CI, 0.4626–2.172; P=0.611; CSS: 99 vs. 60 months; HR, 1.09; 95% CI, 0.402–3.027; P=0.971) (Figure 4).
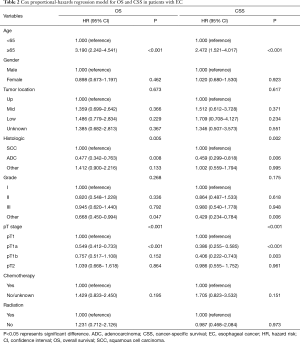
To identify the factors that may influence survival outcomes, we performed multivariate Cox proportional-hazards modeling (Table 2). Age and pT stage were the only two significant factors for OS and CSS (all P<0.05). The other variables including gender, tumor location, histological type, race, grade, pT stage, chemotherapy and RT all did not influence OS and CSS significantly (all P>0.05).
Full table
Discussion
For early stage EC, the efficacy of ER followed by CRT has been evidenced by several studies (5,10,15-20). ER can remove or reduce the size of the tumor to reduce the risk of local recurrence. CRT theoretically can prevent lymph node recurrence. However, the effect of adjuvant RT after ER for early stage EC had previously been validated by very few studies. This retrospective SEER analysis investigated the role of RT after ER in the treatment of early stage EC.
The present study demonstrated that the median survival of the no-radiation group was significantly longer than that of the radiation group. In the T1a stage subgroup, patients who did not receive RT had significantly better OS and CSS outcomes. In T1b and T2 subgroup analysis, both the OS and CSS were not significantly different between the radiation group and the no-radiation group. Furthermore, multivariate regression analysis demonstrated that RT may not influence the OS or CSS significantly after adjustment of significant confounding factors. These results indicated that RT after ER may not benefit T1b and T2 patients and may even lead to poorer treatment outcomes among T1a patients. So far, one study by Ikeda et al had compared adjuvant CRT to observation following endoscopic treatment for high-risk early stage esophageal squamous cell carcinoma (ESCC). Eleven patients underwent CRT radiation and 17 patients were in the observation group. No significant difference in 3-year disease-free survival (DFS) was shown (69% vs. 64%) (21). The result was consistent with our result of T1b subgroup analysis.
Generally, RT can inhibit proliferation of carcinoma cells and reduce the risk of micro-metastasis; thus, it may prevent lymph node recurrence and improve survival when provided after ER. On the other hand, radiation is toxic for normal cells and causes various side effects, increasing the treatment burden for patients. It is likely that the dual effects of RT lead to different treatment outcomes of EC in different stages. The T1a EC patients had low risk of lymph node metastasis, so they might not have benefited greatly from RT. The poorer survival rates in T1a patients who received RT may be attributed to the adverse effect of radiation. The T1b and T2 EC patients were at high risk of metastasis, so RT may have been more effective. However, the included patients were predominantly EAC, which was less sensitive to CRT than ESCC was (22). Furthermore, the patients in these two groups may have had a heavier overall health burden as they were supposed to have surgery but instead underwent ER in most cases due to being unfit for the invasive management. Thus, the adverse effects of RT were strengthened while the positive effects of radiation were compromised. As a result, the T1b and T2 patients receiving RT did not show a significantly better survival rate. Particularly, the T1b EC patients who did not receive RT showed a better 5-year OS rate while T2 EC patients did not show a similar trend, which may correlate to lower risk of lymph node metastasis of T1b EC patients than T2 EC patients.
Therefore, based on the results of the present study, we may conclude that adjuvant RT should be avoided after ER among T1a EC patients; for T1b and T2 EC patients undergoing ER, radiation might be unnecessary as well. However, the present study is a retrospective SEER analysis, so the limitations of retrospective study design make the conclusion not as solid as one made by RCTs. Therefore, well-designed prospective studies, especially RCTs, are warranted to further evaluate the role of RT after ER.
Conclusions
Using SEER data, we revealed that RT after ER did not improve survival in early stage EC patients; specifically, RT conferred no benefit in T1b and T2 patients and may lead to poorer survival in T1a patients. Our findings do not support the addition of RT after ER for early stage EC, especially T1a EC.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. SEER (Surveillance, Epidemiology, and End Results) database is the leading cancer statistics database in the United States and it’s globally open and shared. The authors obtained the data by applying to the authorities.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- NCCN. Esophageal and esophagogastric junction cancers. version 2. 2016. Available online: https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf. Accessed October 11, 2016.
- Yamamoto S, Ishihara R, Motoori M, et al. Comparison between definitive chemoradiotherapy and esophagectomy in patients with clinical stage I esophageal squamous cell carcinoma. Am J Gastroenterol 2011;106:1048-54. [Crossref] [PubMed]
- Motoori M, Yano M, Ishihara R, et al. Comparison between radical esophagectomy and definitive chemoradiotherapy in patients with clinical T1bN0M0 esophageal cancer. Ann Surg Oncol 2012;19:2135-41. [Crossref] [PubMed]
- Goense L, Meziani J, Borggreve AS, et al. Role of adjuvant chemoradiotherapy after endoscopic treatment of early-stage esophageal cancer: a systematic review. Minerva Chir 2018;73:428-36. [PubMed]
- Marino KA, Sullivan JL, Weksler B. Esophagectomy versus endoscopic resection for patients with early-stage esophageal adenocarcinoma: a national cancer database propensity-matched study. J Thorac Cardiovasc Surg 2018;155:2211-8.e1. [Crossref] [PubMed]
- Phoa KN, Pouw RE, Bisschops R, et al. Multimodality endoscopic eradication for neoplastic Barrett oesophagus: results of an European multicentre study (EURO-II). Gut 2016;65:555-62. [Crossref] [PubMed]
- Pech O, May A, Manner H, et al. Long-term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology 2014;146:652-60.e1. [Crossref] [PubMed]
- Shimizu Y, Takahashi M, Yoshida T, et al. Endoscopic resection (endoscopic mucosal resection/ endoscopic submucosal dissection) for superficial esophageal squamous cell carcinoma: current status of various techniques. Dig Endosc 2013;25 Suppl 1:13-9. [Crossref] [PubMed]
- Hamada K, Ishihara R, Yamasaki Y, et al. Efficacy and safety of endoscopic resection followed by chemoradiotherapy for superficial esophageal squamous cell carcinoma: a retrospective study. Clin Transl Gastroenterol 2017;8:e110. [Crossref] [PubMed]
- Schölvinck D, Künzli H, Meijer S, et al. Management of patients with T1b esophageal adenocarcinoma: a retrospective cohort study on patient management and risk of metastatic disease. Surg Endosc 2016;30:4102-13. [Crossref] [PubMed]
- Künzli HT, Belghazi K, Pouw RE, et al. Endoscopic management and follow-up of patients with a submucosal esophageal adenocarcinoma. United European Gastroenterol J 2018;6:669-77. [Crossref] [PubMed]
- Manner H, Pech O, Heldmann Y, et al. The frequency of lymph node metastasis in early-stage adenocarcinoma of the esophagus with incipient submucosal invasion (pT1b sm1) depending on histological risk patterns. Surg Endosc 2015;29:1888-96. [Crossref] [PubMed]
- Gotink AW, Spaander MCW, Doukas M, et al. Exploring diagnostic and therapeutic implications of endoscopic mucosal resection in EUS-staged T2 esophageal adenocarcinoma. Endoscopy 2017;49:941-8. [Crossref] [PubMed]
- Shimizu Y, Kato M, Yamamoto J, et al. EMR combined with chemoradiotherapy: a novel treatment for superficial esophageal squamous-cell carcinoma. Gastrointest Endosc 2004;59:199-204. [Crossref] [PubMed]
- Kawaguchi G, Sasamoto R, Abe E, et al. The effectiveness of endoscopic submucosal dissection followed by chemoradiotherapy for superficial esophageal cancer. Radiat Oncol 2015;10:31. [Crossref] [PubMed]
- Kim HK, Ko WJ, Kwon CI, et al. Endoscopic submucosal dissection followed by concurrent chemoradiotherapy in patients with early esophageal cancer with a high risk of lymph node metastasis. Clin Endosc 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Mochizuki Y, Saito Y, Tsujikawa T, et al. Combination of endoscopic submucosal dissection and chemoradiation therapy for superficial esophageal squamous cell carcinoma with submucosal invasion. Exp Ther Med 2011;2:1065-8. [Crossref] [PubMed]
- Suzuki G, Yamazaki H, Aibe N, et al. Endoscopic submucosal dissection followed by chemoradiotherapy for superficial esophageal cancer: choice of new approach. Radiat Oncol 2018;13:246. [Crossref] [PubMed]
- Uchinami Y, Myojin M, Takahashi H, et al. Prognostic factors in clinical T1N0M0 thoracic esophageal squamous cell carcinoma invading the muscularis mucosa or submucosa. Radiat Oncol 2016;11:84. [Crossref] [PubMed]
- Ikeda A, Hoshi N, Yoshizaki T, et al. Endoscopic submucosal dissection (ESD) with additional therapy for superficial esophageal cancer with submucosal invasion. Intern Med 2015;54:2803-13. [Crossref] [PubMed]
- Shapiro J, van Lanschot JJB, Hulshof MCCM, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]


