
Urine lipoarabinomannan (LAM) and antimicrobial usage in seriously-ill HIV-infected patients with sputum smear-negative pulmonary tuberculosis
Introduction
The urine lipoarabinomannan (LAM) test has been shown not to be useful in untargeted patients with suspected tuberculosis (TB) in a primary health care setting (1), but it is useful for the diagnosis of hospitalized HIV-infected TB patients with a CD4 count <200 cells/mm3, who would otherwise have required further investigation. This is a test based on detection of mycobacterial LAM antigen, a polysaccharide present in mycobacterial cell walls, which is excreted in the urine (2), now available as a point of care urine strip test. Given that between 24% and 62% of HIV-infected patients with TB present with sputum smear-negative findings (3), and that Xpert Mycobacterium tuberculosis (MTB)/rifampicin (RIF) has limited availability in many countries, TB in patients living with advanced stage HIV is often difficult to diagnose. This category of patients frequently have difficulty in producing sputum (4,5), and when combined with low sensitivity of sputum smear microscopy (6,7), leads to delays in diagnosis (8) and treatment. Sputum microbiological culture is the only other definitive tool for the diagnosis of active TB (9), but it is labour intensive and the time to result is prolonged. The difficulty in the diagnosis of sputum smear-negative TB adds to the causes of failure to halt transmission and control the epidemic, especially in high burden countries. Diagnosis is therefore often made on clinical and radiological grounds, which is often atypical (10). Therefore, there is a need for tests that do not require sputum and are more sensitive to ensure early diagnosis.
Urine LAM has been investigated with varying results, but more so in sputum smear-positive patients (1,11-32). There are few studies that have investigated the usefulness of urine LAM in diagnosing sputum smear-negative TB (19,33,34), but none have investigated the use of LAM in reducing antibiotic use. The use of antibiotics to treat suspected TB while waiting for confirmation of diagnosis in hospital is common, although in HIV-infected patients a response to antibiotics may not exclude TB (35). Considering the need to prevent antibiotics resistance, optimal and prudent use of antibiotics is necessary in line with antibiotic stewardship (36,37). In this study, we investigated the diagnostic performance of LAM-ELISA in seriously-ill hospitalized HIV-infected patients with sputum smear-negative pulmonary TB (PTB) and its potential impact on antibiotic usage. The LAM-ELISA, since completion of this study, has been superseded by the LAM lateral flow assay but equivalence between the assays has been demonstrated (18,38).
Methods
Design and setting
This sub-study was conducted as part of a prospective cohort study evaluating the use of a WHO-recommended algorithm to reduce mortality in seriously-ill HIV-infected patients with sputum smear-negative PTB in South Africa (35). Participants were recruited from three hospitals around Durban between March and December 2009.
Participants
Patients with advanced immune suppression and symptoms of TB requiring hospitalization, and with smear-negative sputum, were consecutively enrolled once informed consent was obtained. At one time point on admission, before commencement of antibiotics or anti-TB therapy (ATT), the following samples were collected from consenting participants: spot expectorated sputum and blood for culture, and midstream urine for LAM test. Participants were excluded if they were: (I) currently being treated for latent TB infection (LTBI); (II) treated for TB disease; or (III) were found not to be HIV-infected based on lack of “strong clinical HIV evidence” or an HIV test at either screening, admission or during hospitalization. The study was approved by the Biomedical Research Ethics Committee of the University of KwaZulu-Natal (KZN) (BFC037/08) and an Institutional Review Board at the U.S. Centers for Disease Control and Prevention (CDC).
Patients were started on TB treatment based on criteria defined in the parent study (35): (I) had at least 2 negative specimens for acid fast bacilli; (II) radiographic abnormalities consistent with active TB (according to the treating medical practitioner); (III) laboratory evidence of HIV infection or strong clinical evidence of HIV infection, later confirmed by a laboratory test and (IV) a decision by a clinician to treat with a full course of ATT or sputum smear negative for acid fast bacilli but culture positive for Mycobacterium tuberculosis (MTB).
Sputum and blood processing
All sputum samples were processed using N-acetyl-L-cysteine (NALC)/NaOH and microscopy smear using both auramine and Ziehl-Neelsen staining and were cultured on Middlebrook 7H11 selective agar (Difco) and MGIT liquid medium (Becton Dickenson, Sparks, USA). Positive MTB cultures were confirmed with niacin and nitrate biochemical testing. All blood samples were collected by venipuncture and cultured into aerobic and anaerobic culture systems performed on BacT/ALERT MB (bioMerieux, Marcy-l’Etoile, France) systems.
Urine LAM-ELISA
Urine samples were processed according to the manufacturer’s instructions (Clearview TB® ELISA, Inverness Medical Innovations, Inc., Waltham, USA); within 24 hours of collection urines were boiled at 95–100 °C for 30 min, centrifuged for 15 min at 10,000 rpm and aliquots of 1 mL of the supernatant stored at −70 °C. The assay was performed on the stored aliquots once a week.
From each frozen aliquot, 100 µL sample was incubated for 60 min at ambient temperature, and washed with phosphate buffered saline pH 7.4/Tween-20 (PBST). Subsequently, 100 µL of undiluted conjugate solution (HRP-conjugated LAM-specific rabbit polyclonal antibody) was added. After a 60 min incubation and washing with PBST, 100 µL of the colour developer tetramethylbenzidine (TMB) was administered to each well. The substrate was again incubated for 15 min at ambient temperature, and the reaction was stopped by adding 100 µL of stop solution (1 M H2SO4). The colour development was measured at 450 nm on an automated ELISA plate reader. Samples and negative and positive controls were run in duplicate.
Prior to interpretation of the urine LAM-ELISA results, the validity of each run was confirmed as per the manufacturer’s package insert as: (I) negative control mean optical density (OD) was >0.1 OD and <0.3 OD and (II) positive control mean OD was >0.3 and ≤0.5 above the negative control. Differences for each sample between the two runs were examined to see if their difference were not >15.0%. If the mean OD of the sample aliquots were ≥0.1 above the mean OD of the negative controls, the sample was considered urine LAM positive. LAM-ELISA and the LAM strip test have been found not to be significantly different in studies comparing these two versions (18,38).
Chest radiograph
Chest radiographs (X-rays) were initially read by the treating medical practitioner and then sent to an independent radiologist for reading. The results were then sent to the hospital concerned for the benefit of the medical practitioner. The external radiology results did not directly affect the start of treatment.
Statistical methods
Sample size was not calculated for this sub-study but we used the second cohort of the main study (35). Descriptive statistics were used to summarize the data. Comparisons of categorical data between subgroups were made using Chi-square tests or Fisher’s exact tests as appropriate. Numeric variables such as CD4 and time to event were compared using Mann-Whitney “U” test, while t-test was used for age. Standard measures of diagnostic efficacy are reported where culture is considered the gold standard. Data were entered in Epidata and analysed in Stata V13.1. Kaplan Meier was used to analyse time to death or discharge and a log rank test used to compare subgroups. Median time to death or discharge were compared using Mann-Whitney tests. Statistical analysis was performed using Stata 13 and a P≤0.05 was considered significant.
Results
Demographic characteristics
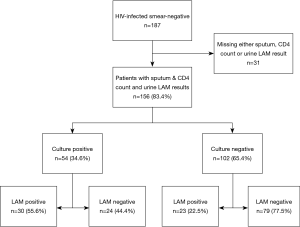
We enrolled a total of 187 patients (Figure 1), out of which 168 had sputum and urine LAM-ELISA results and 156 had all results including CD4 count and were analysed. Table 1 shows the demographic characteristics; 87 (55.8%) patients were male and 154 (98.7%) were black, 81 in WHO stage 3 (51.9%) and 75 (48.1%) in WHO stage 4 (39). Twenty-four (15.4%) were on ART and 43 (27.6%) had a history of prior ATT. Median CD4 count was 75 cells/mm3 [interquartile range (IQR), 34–169 cells/mm3]. Fifty/156 (32.1%) of patients had a CD4 count <50 cells/mm3; 42/156 (26.9%) had CD4 count between 50 and 100 cells/mm3; 35/156 (22.4%) had CD4 count between 100 and 200 cells/mm3; and 29/156 (18.6%) had CD4 count ≥200 cells/mm3.

Full table
Diagnostic accuracy
Out of the 156 patients analysed, 54/156 were sputum culture-positive (34.6%), 53/156 (34.0%) were urine LAM-positive. Thirty urine LAM-positive patients were culture-positive, therefore, the overall test sensitivity was 55.6%. Of the 30 urine LAM-positive/culture positive patients, 21 (70.0%) had CD4 count <100 cells/mm3. Urine LAM sensitivity for patients with CD4 count <100 cells/mm3 was 63.6% (21/33). Urine LAM sensitivity for patients with CD4 count 100–200 cells/mm3 was 64.3% (9/14) and among those with CD4 count >200 cells/mm3, it was 14.3% (1/7). Among the culture negative patients, 23 (22.5%) were urine LAM positive, as seen in Figure 2.
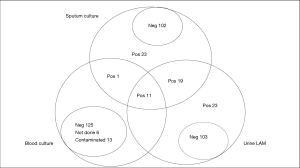
Sputum, chest radiograph, urine LAM and blood culture
Figure 2 shows culture positivity, with 54/156 (34.6%) sputum culture positive patients with at least one sputum sample. Although all patients’ diagnosis included chest radiography, only 120/156 (76.9%) patients had their chest X-rays read by an independent radiologist. Of the 53 urine LAM-positive patients 35/53 (66.0%) had a chest X-ray read by the radiologist and 20/35 (57.1%) were consistent with PTB. The majority, 52/53 (98.1%), of the urine LAM-positive patients had blood culture ordered, 11/52 (21.2%) of whom were sputum and blood culture positive and 32/52 (61.5%) blood culture negative, with 7/52 (13.5%) contaminated and 2/52 (3.8%) not done. Only 1/54 (1.9%) culture positive patient was urine LAM negative and 8/11 (72.7%) blood culture positive patients had a CD4 ≤100 cells/mm3. As shown in Table 2, of the 12 blood culture positive patients, 6 (50.0%) were confirmed as not PTB by the radiologist.
Full table
Factors associated with a positive urine LAM result
Prior ATT was associated with a negative urine LAM result at the univariate level, 8 (15.1%) of urine-LAM positive patients had prior ATT compared to 35 (34.0%) of urine-LAM negative patients (OR: 2.6; 95% CI: 1.2–5.9, P=0.02). A positive urine LAM result was also associated with a CD4 count <200 cells/mm3, 39.4% (50/127); compared to 10.3% (3/29) patients with CD4 count >200 cells/mm3 (OR: 5.6; 95% CI: 1.6–19.6, P=0.007). In a multivariable model containing variables significant at the univariate level, both remained independently associated with a positive LAM result: ORadj: 3.2; 95% CI: 1.3–7.6, P=0.009; ORadj: 6.2; 95% CI: 1.8–21.8, P=0.005) respectively.
Antibiotic use and ATT
The majority 48/53 (90.6%) of the urine LAM-positive patients received antibiotics and 18/46 (39.1%) with recorded time, were on antibiotics for a median time of 5 days (Table 3). Of the 48/53 (90.6%) LAM-positive patients that received antibiotics, 10 (20.8%) received three types or more while 23/48 (47.9%) received two types and 15/48 (31.3%) received one type of antibiotics. All patients received ATT, of the 53 urine LAM-positive patients, 32 (60.4%) received ATT within 3 days of admission as per protocol, and the rest within 4–22 days.

Full table
Treatment outcomes—time to discharge and time to death
Median time to discharge, excluding deaths was 5 days (IQR, 4–6 days) for the urine LAM positive (n=48) and 6 days (IQR, 4–8 days) for the urine LAM negatives (n=99), and was statistically significantly different (P=0.03). Median time to death was 12 days (IQR, 11–13 days) for the urine LAM positive (n=5) and 7 days (IQR, 7–8 days), for the urine LAM negative (n=4) but was not significantly different (P=0.11).
Discussion
We evaluated the use of urine LAM for the diagnosis of smear negative TB and found that more than 52% of culture-positive patients were urine LAM positive. Moreover, nearly 20% of sputum smear- and culture-negative presumptive TB patients were urine LAM positive.
Almost all the patients in this study received empiric antibiotics. With the availability of LAM results, in at least one third of the patients, antibiotic usage could have been obviated or curtailed. Although all patients in this study should have been started on ATT within 3 days, (as per 2007 WHO guidelines for treatment of smear negative TB and study protocol), and all were found to have chest X-ray abnormality consistent with TB by their treating clinician, we found that at least 10 LAM-positive patients were given three or more types of antibiotics with or without ATT for more than 5 days (43.5%). This was to empirically cover the patients for other bacterial infections given that the TB could not be rapidly confirmed (however, there were no confirmed bacterial infections confirmed in these patients). In addition, nearly 40% of the 53 urine-LAM positive patients received ATT within 4–22 days, and the delay may have been due to the need to allow antibiotics time to act while waiting for sputum-culture results.
It is reasonable to assume that fewer antibiotics could have been used had the urine LAM results been available on the day of admission. Peter et al. suggested that the urine LAM assay may be used as a “rule in” test for smear negative HIV-infected patients (40). Using LAM as a rule in test, in conjunction with clinical signs and symptoms and radiographic findings, urine LAM-positive patients could receive ATT within hours of admission, saving excessive use of antibiotics. This would be in line with current country-specific and antibiotic stewardship guidelines. Indeed, Padayatchi et al. have advocated for ensuring that patients receive appropriate treatment with specific attention to dosing and duration as the first goal of antibiotic stewardship preventing drug overuse and abuse as the second goal, and minimizing TB drug resistance at an individual and community level as the third goal (41). However, clinicians in many settings still rely strongly on antibiotic trials for smear negative TB (42). There are recent studies investigating the use of trial of antibiotics as a diagnostic tool for smear negative TB (43) indicating that the practice is ongoing. A study by Walusimbi et al. (44) recommends that it is useful to start HIV infected smear negative patients on an initial course of antibiotics and in South Africa, four of the five patients given antibiotics while waiting for culture results (patients were smear and Xpert negative) could not be started on ATT as they were lost to follow up by the time the results were available, and recommended to start HIV-infected sputum smear-negative patients on an initial course of antibiotics (45). In this setting, a positive urine LAM test is likely to reduce antibiotic usage.
We found that a positive blood culture was one of the factors associated with a positive urine LAM, consistent with findings of Shah and colleagues (20). More than 80% of patients with positive blood culture were positive for urine LAM, and 58% of them had chest X-rays not consistent with TB by a radiologist (Table 2). Thus, urine LAM, being of much lower cost and a point of care assay, could replace blood culture as a screening investigation in HIV-infected hospitalised patients with fever and advanced HIV disease.
Our study had several limitations. We did not enroll a control group, and since all the patients were either culture-confirmed TB or clinically diagnosed TB, we could not calculate the specificity. Indeed, a limited autopsy study by Cohen et al. found that almost half of the HIV coinfected inpatients who died had evidence of TB at the time of death (46). More than half were not on TB treatment, and based on this information from the same geographical area as our study, we could not confirm that the culture negative patients did not have TB disease. Nevertheless, it was interesting that LAM detected 23 additional diagnoses in the culture negative empirically treated group, which represents a 14.7% increase over the number of cases initially diagnosed. The LAM-ELISA, since completion of this study, has been superseded by the LAM lateral flow assay but equivalence between the assays has been demonstrated (18,38). Interestingly, there are new assays emerging with better sensitivity. The Fujifilm SILVAMP TB LAM assay offered superior diagnostic sensitivity compared to urine LAM, while maintaining specificity (47). We did not directly demonstrate a reduction in antibiotic usage though it is likely this could have been substantially curtailed in urine LAM-positive patients. This will need to be evaluated in a future prospective study using LAM and remains an important question to be answered.
In summary, urine LAM testing in sputum smear-negative severely ill HIV-infected hospitalized patients with suspected TB and advanced immunosuppression offers the potential to start ATT early and to decrease the number and duration of antibiotics used.
Acknowledgments
We thank the patients who took part in the study and the staff that assisted in it. We also thank the South African Department of Health, particularly the staff and patients of the three participating hospitals; the national tuberculosis programme director, Lindiwe Mvusi; and the provincial tuberculosis programme director, Bruce Margot.
Funding: K Dheda is supported by the South African MRC (RFA-EMU-02-2017) and the EDCTP (TMA-2015SF-1043 & TMA- 1051-TESAII).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Biomedical Research Ethics Committee of the University of KwaZulu-Natal (KZN) (BFC037/08) and an Institutional Review Board at the U.S. Centers for Disease Control and Prevention (CDC). Written informed consent was obtained from all patients for publication of this manuscript and any accompanying images.
References
- Dheda K, Davids V, Lenders L, et al. Clinical utility of a commercial LAM-ELISA assay for TB diagnosis in HIV-infected patients using urine and sputum samples. PLoS One 2010;5:e9848. [Crossref] [PubMed]
- Hamasur B, Bruchfeld J, Van Helden P, et al. A sensitive urinary lipoarabinomannan test for tuberculosis. PLoS One 2015;10:e0123457. [Crossref] [PubMed]
- Getahun H, Harrington M, O'Brien R, et al. Diagnosis of smear-negative pulmonary tuberculosis in people with HIV infection or AIDS in resource-constrained settings: informing urgent policy changes. Lancet 2007;369:2042-9. [Crossref] [PubMed]
- Vargas D, Garcia L, Gilman RH, et al. Diagnosis of sputum-scarce HIV-associated pulmonary tuberculosis in Lima, Peru. Lancet 2005;365:150-2. [Crossref] [PubMed]
- Peter JG, Theron G, Muchinga TE, et al. The diagnostic accuracy of urine-based Xpert MTB/RIF in HIV-infected hospitalized patients who are smear-negative or sputum scarce. PLoS One 2012;7:e39966. [Crossref] [PubMed]
- Swai HF, Mugusi FM, Mbwambo JK. Sputum smear negative pulmonary tuberculosis: sensitivity and specificity of diagnostic algorithm. BMC Res Notes 2011;4:475. [Crossref] [PubMed]
- Cattamanchi A, Dowdy DW, Davis JL, et al. Sensitivity of direct versus concentrated sputum smear microscopy in HIV-infected patients suspected of having pulmonary tuberculosis. BMC Infect Dis 2009;9:53. [Crossref] [PubMed]
- Lee HM, Shin JW, Kim JY, et al. HRCT and whole-blood interferon-gamma assay for the rapid diagnosis of smear-negative pulmonary tuberculosis. Respiration 2010;79:454-60. [Crossref] [PubMed]
- Lange C, Mori T. Advances in the diagnosis of tuberculosis. Respirology 2010;15:220-40. [Crossref] [PubMed]
- Burman WJ, Jones BE. Clinical and radiographic features of HIV-related tuberculosis. Semin Respir Infect 2003;18:263-71. [Crossref] [PubMed]
- Daley P, Michael JS, Hmar P, et al. Blinded evaluation of commercial urinary lipoarabinomannan for active tuberculosis: a pilot study. Int J Tuberc Lung Dis 2009;13:989-95. [PubMed]
- Tessema TA, Bjune G, Hamasur B, et al. Circulating antibodies to lipoarabinomannan in relation to sputum microscopy, clinical features and urinary anti-lipoarabinomannan detection in pulmonary tuberculosis. Scand J Infect Dis 2002;34:97-103. [Crossref] [PubMed]
- Tessema TA, Bjune G, Assefa G, et al. Clinical and radiological features in relation to urinary excretion of lipoarabinomannan in Ethiopian tuberculosis patients. Scand J Infect Dis 2002;34:167-71. [Crossref] [PubMed]
- Sada E, Aguilar D, Torres M, et al. Detection of lipoarabinomannan as a diagnostic test for tuberculosis. J Clin Microbiol 1992;30:2415-8. [PubMed]
- Boehme C, Molokova E, Minja F, et al. Detection of mycobacterial lipoarabinomannan with an antigen-capture ELISA in unprocessed urine of Tanzanian patients with suspected tuberculosis. Trans R Soc Trop Med Hyg 2005;99:893-900. [Crossref] [PubMed]
- Pereira Arias-Bouda LM, Nguyen LN, Ho LM, et al. Development of Antigen Detection Assay for Diagnosis of Tuberculosis Using Sputum Samples. J Clin Microbiol 2000;38:2278-83. [PubMed]
- Minion J, Leung E, Talbot E, et al. Diagnosing tuberculosis with urine lipoarabinomannan: systematic review and meta-analysis. Eur Respir J 2011;38:1398-405. [Crossref] [PubMed]
- Lawn SD, Kerkhoff AD, Vogt M, et al. Diagnostic accuracy of a low-cost, urine antigen, point-of-care screening assay for HIV-associated pulmonary tuberculosis before antiretroviral therapy: a descriptive study. Lancet Infect Dis 2012;12:201-9. [Crossref] [PubMed]
- Gounder CR, Kufa T, Wada NI, et al. Diagnostic accuracy of a urine lipoarabinomannan enzyme-linked immunosorbent assay for screening ambulatory HIV-infected persons for tuberculosis. J Acquir Immune Defic Syndr 2011;58:219-23. [Crossref] [PubMed]
- Shah M, Variava E, Holmes CB, et al. Diagnostic accuracy of a urine lipoarabinomannan test for tuberculosis in hospitalized patients in a High HIV prevalence setting. J Acquir Immune Defic Syndr 2009;52:145-51. [Crossref] [PubMed]
- Mutetwa R, Boehme C, Dimairo M, et al. Diagnostic accuracy of commercial urinary lipoarabinomannan detection in African tuberculosis suspects and patients. Int J Tuberc Lung Dis 2009;13:1253-9. [PubMed]
- Tessema TA, Hamasur B, Bjun G, et al. Diagnostic evaluation of urinary lipoarabinomannan at an Ethiopian tuberculosis centre. Scand J Infect Dis 2001;33:279-84. [Crossref] [PubMed]
- Mutetwa R, Boehme C, Dimairo M, et al., editors. Evaluation of a commercial urine lipoarabinomannan ELISA kit for diagnosis of TB in a high HIV prevalence setting. 16th Conference on Retroviruses and Opportunistic infections, 2009.
- Sada E, Brennan PJ, Herrera T, et al. Evaluation of lipoarabinomannan for the serological diagnosis of tuberculosis. J Clin Microbiol 1990;28:2587-90. [PubMed]
- Wood R, Racow K, Bekker LG, et al. Lipoarabinomannan in urine during tuberculosis treatment: association with host and pathogen factors and mycobacteriuria. BMC Infect Dis 2012;12:47. [Crossref] [PubMed]
- Briken V, Porcelli SA, Besra GS, et al. Mycobacterial lipoarabinomannan and related lipoglycans: from biogenesis to modulation of the immune response. Mol Microbiol 2004;53:391-403. [Crossref] [PubMed]
- Chatterjee D, Khoo KH. Mycobacterial lipoarabinomannan: an extraordinary lipoheteroglycan with profound physiological effects. Glycobiology 1998;8:113-20. [Crossref] [PubMed]
- Lawn SD. Point-of-care detection of lipoarabinomannan (LAM) in urine for diagnosis of HIV-associated tuberculosis: a state of the art review. BMC Infect Dis 2012;12:103. [Crossref] [PubMed]
- Shah M, Martinson NA, Chaisson RE, et al. Quantitative analysis of a urine-based assay for detection of lipoarabinomannan in patients with tuberculosis. J Clin Microbiol 2010;48:2972-4. [Crossref] [PubMed]
- Hamasur B, Bruchfeld J, Haile M, et al. Rapid diagnosis of tuberculosis by detection of mycobacterial lipoarabinomannan in urine. J Microbiol Methods 2001;45:41-52. [Crossref] [PubMed]
- Strohmeier GR, Fenton MJ. Roles of lipoarabinomannan in the pathogenesis of tuberculosis. Microbes Infect 1999;1:709-17. [Crossref] [PubMed]
- Lawn SD, Edwards DJ, Kranzer K, et al. Urine lipoarabinomannan assay for tuberculosis screening before antiretroviral therapy diagnostic yield and association with immune reconstitution disease. AIDS 2009;23:1875-80. [Crossref] [PubMed]
- Drain PK, Gounder L, Sahid F, et al. Rapid Urine LAM Testing Improves Diagnosis of Expectorated Smear-Negative Pulmonary Tuberculosis in an HIV-endemic Region. Sci Rep 2016;6:19992. [Crossref] [PubMed]
- Nakiyingi L, Ssengooba W, Nakanjako D, et al. Predictors and outcomes of mycobacteremia among HIV-infected smear- negative presumptive tuberculosis patients in Uganda. BMC Infect Dis 2015;15:62. [Crossref] [PubMed]
- Holtz TH, Kabera G, Mthiyane T, et al. Use of a WHO-recommended algorithm to reduce mortality in seriously ill patients with HIV infection and smear-negative pulmonary tuberculosis in South Africa: an observational cohort study. Lancet Infect Dis 2011;11:533-40. [Crossref] [PubMed]
- Bell BG, Schellevis F, Stobberingh E, et al. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect Dis 2014;14:13. [Crossref] [PubMed]
- Murphy DM, Hanchett M, Olmsted RN, et al. Competency in infection prevention: a conceptual approach to guide current and future practice. Am J Infect Control 2012;40:296-303. [Crossref] [PubMed]
- Peter JG, Theron G, van Zyl-Smit R, et al. Diagnostic accuracy of a urine lipoarabinomannan strip-test for TB detection in HIV-infected hospitalised patients. Eur Respir J 2012;40:1211-20. [Crossref] [PubMed]
- WHO. WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children. WHO, 2007.
- Peter J, Green C, Hoelscher M, et al. Urine for the diagnosis of tuberculosis: current approaches, clinical applicability, and new developments. Curr Opin Pulm Med 2010;16:262. [Crossref] [PubMed]
- Padayatchi N, Mahomed S, Loveday M, et al. Antibiotic stewardship for drug resistant tuberculosis. Expert Opin Pharmacother 2016;17:1981-3. [Crossref] [PubMed]
- McDowell A, Pai M. Treatment as diagnosis and diagnosis as treatment: empirical management of presumptive tuberculosis in India. Int J Tuberc Lung Dis 2016;20:536-43. [Crossref] [PubMed]
- Divala TH, Fielding KL, Nliwasa M, et al. Sensitivity and specificity of using trial-of-antibiotics versus sputum mycobacteriology for diagnosis of tuberculosis: protocol for a systematic literature review. Syst Rev 2018;7:141. [Crossref] [PubMed]
- Walusimbi S, Semitala F, Bwanga F, et al. Outcomes of a clinical diagnostic algorithm for management of ambulatory smear and Xpert MTB/Rif negative HIV infected patients with presumptive pulmonary TB in Uganda: a prospective study. Pan Afr Med J 2016;23:154. [PubMed]
- Van Rie A, Page-Shipp L, Hanrahan CF, Schnippel K, Dansey H, Bassett J, et al. Point-of-care Xpert(R) MTB/RIF for smear-negative tuberculosis suspects at a primary care clinic in South Africa. Int J Tuberc Lung Dis 2013;17:368-72. [Crossref] [PubMed]
- Cohen T, Murray M, Wallengren K, et al. The prevalence and drug sensitivity of tuberculosis among patients dying in hospital in KwaZulu-Natal, South Africa: a postmortem study. PLoS Med 2010;7:e1000296. [Crossref] [PubMed]
- Broger T, Sossen B, du Toit E, et al. Novel High Sensitivity Tuberculosis Point-of-Care Test for People Living with HIV. 2018. Available online: https://ssrn.com/abstract=3254479


