The use of surgery in a real-world clinic to diagnose and treat pulmonary cryptococcosis in immunocompetent patients
Introduction
Pulmonary cryptococcosis (PC) is a fungal infection caused by inhalation of spores of Cryptococcus neoformans and C. gattii, which can trigger pulmonary involvement (1). Inhalation of cryptococcal particles can cause pulmonary infection, and subsequent hematogenous dissemination may trigger central nervous system infection, especially in immunocompromised patients (2). Although PC is more common in immunocompromised patients, including acquired immune deficiency syndrome (AIDS) patients, solid-organ transplant recipients, those with hematological malignancies, and patients on chronic corticosteroids or other immunosuppressive therapies (3-5), PC is occasionally seen in immunocompetent patients (6). The incidence of PC in such patients is increasing, most of whom have no apparent underlying disease (7,8).
In immunocompetent hosts, PC often mimics a lung malignancy, or a chronic inflammatory or infectious disease such as tuberculosis. On imaging, single or multiple nodular lesions or mass-like consolidations are apparent (9,10). Therefore, the diagnostic approach varies widely depending on the initial presumed diagnosis and radiological findings. If the presumed diagnosis is benign, physicians may prefer to use non-surgical modalities, such as percutaneous needle biopsy (PCNB) or transbronchial lung biopsy (TBLB), for definitive diagnosis. If the presumed diagnosis is malignant, more invasive diagnostic modalities such as video-assisted thoracoscopic surgery (VATS) are employed, especially when the lesions are not peripheral. However, previous studies have simply described the diagnostic modalities employed; the factors that influence the diagnostic approaches chosen remain unclear.
Various PC treatment options are available, including surgical resection, antifungal agents, and observation (11,12). However, as surgical resection is required for the diagnosis of many PC cases, treatment options should be determined on the basis of the need for such resection. No specific recommendations for the treatment of PC diagnosed by surgical resection are available and it is unclear whether such patients require additional antifungals. Also, the natural disease course in such patients has not been well-documented. Thus, we evaluated the role for surgery in PC diagnosis and treatment in a real-world clinic, and the need (or otherwise) for additional antifungals after surgical resection of PC.
Methods
Patients
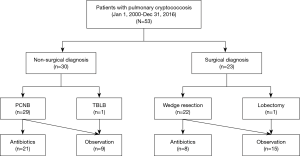
The medical records of 53 immunocompetent patients diagnosed with PC in Samsung Medical Center (a 1,979-bed referral hospital) in Seoul, South Korea, between January 2000 and December 2016, were retrospectively reviewed. All patients had pathologically confirmed PC; biopsy specimens were obtained via non-surgical modalities such as PCNB (n=29, 55%) and TBLB (n=1, 2%) and a surgical modality (VATS) (n=23, 43%) (Figure 1). The study was approved by the Institutional Review Board of Samsung Medical Center. The need for written patient consent was waived because the study was retrospective in nature (IRB no. 2017-06-142-011).
Image definition and interpretation
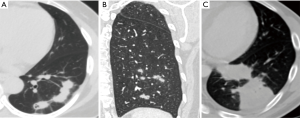
Thoracic computed tomography (CT) images were evaluated for the presence of lung abnormalities (nodules, masses, and consolidations). All radiological data were retrospectively reviewed by the three co-authors (B Yang, MY Kim, and KS Lee). Nodules were defined as round or oval opacities <3 cm in the greatest diameter; larger opacities were termed masses. A consolidation was defined as a parenchymal non-nodular opacity that obscured the underlying pulmonary architecture and that was often accompanied by an air bronchogram. Each pattern was classified as predominantly central, peripheral, undetermined, or multiple. Undetermined means predominantly lesion defined as lesion between central and peripheral, and multiple is defined as involve of more than two of peripheral, central, and undetermined. Cavitations within nodules or masses were noted. After assessment of lung parenchymal abnormalities, all lesions were further classified into six patterns: solitary pulmonary nodules, solitary pulmonary masses, scattered nodules, clustered nodules, a bronchopneumonic pattern, and a mixed pattern. Solitary pulmonary nodes and masses were defined when the lung lesions consisted of a single nodule or a single mass. The clustered nodular pattern was defined when the lesions consisted of multiple nodules of various sizes in segments of the same lobe (Figure 2A). The scattered nodular pattern was defined when the lesions consisted of multiple nodules of various sizes in segments of the same lung (Figure 2B). The bronchopneumonic pattern (Figure 2C) was defined when lung lesions were comprised of areas of lobular, subsegmental, or segmental consolidation, when the lesions featured ill-defined small centrilobular nodules (4–10 mm in diameter, reflecting peribronchiolar consolidation), or when the lesions had a “tree-in-bud” appearance (small centrilobular nodules with branching nodules within secondary pulmonary lobules) (13).
Pathologic diagnosis of Cryptococcus
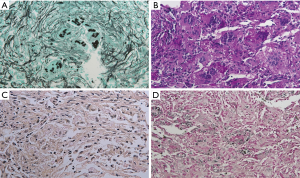
In immunocompetent patients, the host response to Cryptococcus is predominantly granulomatous inflammation or granuloma, which is accompanied by various degrees of fibrosis and necrosis (14). The histologic diagnosis of Cryptococcus was based on its typical morphology of narrow-based budding yeasts (4–10 µm in size) with a thick mucicarmine positive capsule (Figure 3A,B,C). All cases of our study were performed GMS (Gomori Methenamine-Silver), PAS (Periodic Acid Schiff) and mucicarmine staining. In some case with lesser amount of characteristic capsule, the Fontana Masson stain was performed to discriminate other yeasts of similar size, such as candida or histoplasma. The characteristic of this stain was that only cryptococci, which contain melanin, showed positive, so we could distinguish cryptococci from other yeasts (Figure 3D).
Treatments and responses
After PC diagnosis, the treatment modalities were applied at the discretion of the attending physicians. Generally, patients who were diagnosed non-surgically were given antifungals or followed-up without specific treatment, while patients who were diagnosed surgically were further treated with antifungals or followed-up without additional antifungal treatment. After initial treatment, patients were usually followed-up at 1-month intervals for 3 months, and 3-month intervals thereafter, via chest X-ray or CT in addition to laboratory tests. The treatment responses were categorized as complete or partial, stable disease, or progressive disease, as described previously. A complete response was defined as complete disappearance of all clinical symptoms and radiological findings. A partial response was defined as partial resolution of the chest CT lesion, regardless of clinical improvement. No change was defined as no imaging change and progressive disease was defined as progression evident on chest CT (15).
Statistical analysis
All data are presented as medians with interquartile ranges (IQRs) for continuous variables, and as numbers with percentages for categorical variables. Data were compared using the Mann-Whitney U test for continuous variables and the Pearson χ2 test or Fisher’s exact test for categorical variables. The one-sample proportion test was used to derive P values for the radiological findings. A two-sided P<0.05 was considered to reflect statistical significance in all analyses. All statistical analyses were performed using SPSS statistical software (ver. 23.0; IBM Corp., Armonk, NY, USA) and R (ver. 3.2.3; R Foundation for Statistical Computing, Vienna, Austria).
Results
Patients
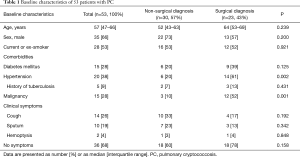
The baseline characteristics of the 53 PC patients are summarized in Table 1. They included 35 males (66%), and the median patient age was 57 years (IQR: 47–66 years). Twenty-eight patients (53%) were current or ex-smokers. The most common comorbidities were hypertension (n=20, 38%), a malignancy (n=15, 28%), diabetes mellitus (n=15, 28%), and a previous history of tuberculosis (n=5, 9%). In terms of symptoms at the time of PC diagnosis, 36 patients (68%) had no symptoms, 14 (26%) had cough, 10 (19%) exhibited sputum production, and 2 (4%) exhibited hemoptysis.
Full table
As shown in Figure 1, 30 patients (57%) were diagnosed using non-surgical modalities including PCNB (n=29) and TBLB (n=1), and 23 were diagnosed using surgical modalities including VATS wedge resection (n=22) and VATS lobectomy (n=1). As shown in Table 1, there was no significant difference in age, sex, smoking history, the frequency of comorbidities such as diabetes mellitus, history of previous tuberculosis, or clinical symptoms between patients diagnosed surgically and non-surgically. However, the frequency of malignancy was significantly higher in patients diagnosed surgically [52% (12/23) vs. 10% (3/30); P=0.001].
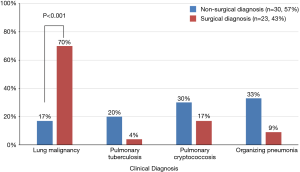
As shown in Figure 4, the initial presumed clinical diagnosis in surgically diagnosed patients was more commonly lung malignancy versus those who were non-surgically diagnosed [70% (16/23) vs. 17% (5/30); P<0.001 for initial clinical diagnosis].
Radiological findings
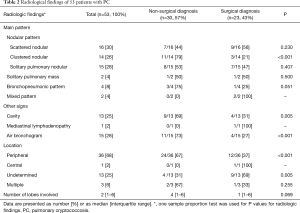
The thoracic CT findings are summarized in Table 2. In terms of radiological patterns, the scattered nodular pattern was the most common (n=16, 30%), followed by the solitary nodular pattern (n=15, 28%), the clustered nodular pattern (n=10, 19%), the multiply clustered nodular pattern (n=4, 8%), the bronchopneumonic pattern (n=4, 8%), and the mixed pattern (n=2, 3%). Cavitation was found in 13 patients (25%), and air bronchograms was seen in 15 (28%). Of all lung lesions, 66% (n=35) were peripheral. Radiological characteristics of 15 cryptococcosis patients with a solitary pulmonary pattern show in Table S1. There was no difference of the radiological characteristics in the choice of diagnosis modality among patients with solitary pulmonary nodules. Also, the initial clinical diagnosis and radiological findings according to the diagnostic approach were showed in Table S2.
Full table

Full table
Full table
As shown in Table 2, for the diagnosis of PC, surgical modality was more frequently used than non-surgical modality in patients with the following radiologic patterns; clustered nodular pattern (79% vs. 21%; P<0.001), cavities (69% vs. 31%; P=0.005), air bronchograms (73% vs. 27%; P<0.005), and peripheral location (67% vs. 33%; P<0.001).
Treatments and responses
Comparison of treatment responses between non-surgically diagnosed patients and surgically diagnosed patients
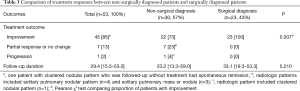
Of the 30 patients who were non-surgically diagnosed, 21 were treated with antifungals including fluconazole (n=19) and itraconazole (n=2) for a median of 6.3 months (IQR, 3.6–6.5 months) and 9 were followed-up without treatment. In comparison, of 23 patients who were surgically diagnosed, 8 were treated with antifungals including fluconazole (n=7) and itraconazole (n=1) for a median of 5.6 months (IQR, 3.0–7.0 months) (Figure 1). As shown in Table 3, During a median of 29.4 months follow-up duration, the proportion of patients who had improvement were significantly higher in patients who were surgically diseased than those who were non-surgically diagnosed [100% (23/23) vs. 73% (22/57); P=0.007]. There were no casas with recurrent PC after complete improvement. Also, none of those who underwent surgical resection had post-operative complications.
Full table
Comparison of clinical characteristics and treatment responses between patients diagnosed surgically who did and did not receive additional antifungal treatment
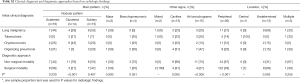
As shown in Table 4, there was no significant difference in age, sex, smoking history, comorbidity status, or radiological findings between patients who did and did not receive antifungals after surgical resection (Table 4). Regardless of antifungal treatment status, no patient who underwent surgical resection developed progressive disease.
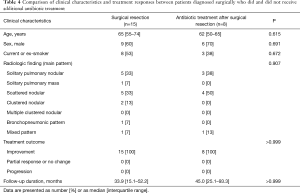
Full table
Discussion
In this study, most PC cases were found incidentally, and thus in the absence of specific symptoms, in most immunocompetent subjects. The diagnostic approach was affected by several factors including the initial presumed clinical diagnoses and the radiological patterns. Surgical resection was required to diagnose PC in 43% of patients. The proportion of patients with an initial presumed diagnosis of lung malignancy was significantly higher among surgically diagnosed than non-surgically diagnosed patients. Whereas undetermined location was more common in surgically diagnosed patients, radiologic findings including clustered nodular pattern, cavities, air bronchograms, and peripherally located lesions were more common among non-surgically diagnosed patients. Additionally, we found that antifungals were not necessary to prevent PC recurrence after complete surgical resection.
The most common lung abnormality patterns were clustered or solitary pulmonary nodules (3,10,13,16). In agreement with these findings, the most frequent lung abnormality patterns detected by CT in the present study were nodular (84%), including the scattered nodular pattern (30%), the solitary pulmonary nodular pattern (28%), and the clustered nodular pattern (26%). Extending the findings of previous studies, we also found that the chosen diagnostic approach was significantly affected by these radiological patterns. Most patients with clustered nodular patterns were diagnosed non-surgically, whereas only about half of those with scattered or solitary nodular patterns were diagnosed non-surgically. Several possible explanations for these results may be proposed. As the clustered nodular pattern is the best-known PC radiological pattern, non-surgical methods might have been preferred because of initial suspicion of PC in 43% of patients. Also, most clustered nodular lesions were peripheral (79%), and thus easy to approach via PCNB. In contrast, the scattered nodular patterns might not have been considered suggestive of PC; PC was very rarely considered as a presumed diagnosis in such patients. Moreover, as the lesions were scattered throughout the lobes, PCNB may not have been feasible. However, in some cases, PC was successfully diagnosed using non-invasive modalities such as TBLB and PCNB. Thus, clinical suspicion of PC in cases exhibiting the scattered nodular pattern might reduce the rate of unnecessary surgical resection. In cases with solitary pulmonary nodules, the most frequent presumed clinical diagnosis was a malignancy. Therefore, some cases, especially those exhibiting high-level FDG uptake, may have undergone surgical resection under a high suspicion of malignancy. Also, radiological findings such as cavities and air bronchograms were associated with the use of non-surgical diagnostic approaches.
PC treatment in immunocompetent patients remains controversial. Treatments include simple observation, medical treatment, and surgical resection. A few reports (15,17,18) found that PC in symptom-free immunocompetent patients was indolent and resolved spontaneously. However, in our study, of nine patients who were followed-up without specific treatment, only one exhibited a complete response, no changes were evident in seven, and one progressed. Therefore, we suggest that even patients who were previously healthy (thus without underlying disease) should be treated rather than simply observed to prevent unnecessary patient concern and the inevitable radiological exposure associated with close follow-up.
In terms of surgical resection, a significant proportion of patients required diagnostic resection, as is also true in patients with pulmonary actinomycosis (19) and pulmonary MALT lymphoma (20,21), which mimic lung cancer. However, as is also true of these rare diseases, no guidelines for PC treatment diagnosed by surgical resection are available. Several studies (6,15) have reported complete or partial responses to fluconazole after PC diagnosis via surgical resection or PCNA. A recent study (22) recommended that patients with proven or probable PC, whether symptomatic or not, should receive a single course of antifungal therapy. However, no study has yet evaluated the role played by antifungal treatment after surgical resection. Thus, the fact that antifungals are not required constitutes new and useful information; this is also the case with other rare diseases (19-21).
Our study had several limitations. First, the work was retrospective in nature and was performed in a single center. Second, although we showed that complete resection prevented PC recurrence without the need for antifungals after surgical resection, the number of patients in the two groups was relatively small. Nevertheless, we suggest that complete surgical resection prevents recurrence in immunocompetent patients with localized lesions compatible with PC. In such cases, surgical resection not only allows accurate diagnosis, but also successful management (and thus a high probability of complete response). However, further studies with longer-term follow-up are needed. Third, we did not have culture results. Thus, we could not identify subspecies of Cryptococcus. Thus, future studies are needed to evaluate whether the clinical and radiological manifestations are different according to subtypes of Cryptococcus.
Conclusions
In conclusion, we found that surgical resection was diagnostically required by our PC patients. Initial clinical suspicion of a lung malignancy was associated with surgical diagnosis. Whereas undetermined location was associated with surgical diagnostic approaches, the presence of the clustered nodular pattern, cavities, air bronchograms, and peripheral lesional locations was associated with non-surgical diagnostic approaches. Surgical resection not only afforded reliable diagnoses but also effectively treated PC. Antifungals were unnecessary after complete surgical resection.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Review Board of Samsung Medical Center (IRB no. 2017-06-142-011). The need for written patient consent was waived because the study was retrospective in nature.
References
- Perfect JR, Dismukes WE, Dromer F, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america. Clin Infect Dis 2010;50:291-322. [Crossref] [PubMed]
- Sarosi GA. Cryptococcal pneumonia. Semin Respir Infect 1997;12:50-3. [PubMed]
- Chang WC, Tzao C, Hsu HH, et al. Pulmonary cryptococcosis: comparison of clinical and radiographic characteristics in immunocompetent and immunocompromised patients. Chest 2006;129:333-40. [Crossref] [PubMed]
- Singh N, Alexander BD, Lortholary O, et al. Pulmonary cryptococcosis in solid organ transplant recipients: clinical relevance of serum cryptococcal antigen. Clin Infect Dis 2008;46:e12-8. [Crossref] [PubMed]
- Liu K, Ding H, Xu B, et al. Clinical analysis of non-AIDS patients pathologically diagnosed with pulmonary cryptococcosis. J Thorac Dis 2016;8:2813-21. [Crossref] [PubMed]
- Choi KH, Park SJ, Min KH, et al. Treatment of asymptomatic pulmonary cryptococcosis in immunocompetent hosts with oral fluconazole. Scand J Infect Dis 2011;43:380-5. [Crossref] [PubMed]
- Liu YN, She DY, Sun TY, et al. A multicentre retrospective study of pulmonary mycosis clinically proven from 1998 to 2007. Zhonghua Jie He He Hu Xi Za Zhi 2011;34:86-90. [PubMed]
- Chen S, Sorrell T, Nimmo G, et al. Epidemiology and host- and variety-dependent characteristics of infection due to Cryptococcus neoformans in Australia and New Zealand. Australasian Cryptococcal Study Group. Clin Infect Dis 2000;31:499-508. [Crossref] [PubMed]
- Khoury MB, Godwin JD, Ravin CE, et al. Thoracic cryptococcosis: immunologic competence and radiologic appearance. AJR Am J Roentgenol 1984;142:893-6. [Crossref] [PubMed]
- Lindell RM, Hartman TE, Nadrous HF, et al. Pulmonary cryptococcosis: CT findings in immunocompetent patients. Radiology 2005;236:326-31. [Crossref] [PubMed]
- Aberg JA, Mundy LM, Powderly WG. Pulmonary cryptococcosis in patients without HIV infection. Chest 1999;115:734-40. [Crossref] [PubMed]
- Igai H, Gotoh M, Yokomise H. Computed tomography (CT) and positron emission tomography with 18Ffluoro-2-deoxy-D-glucose (FDG-PET) images of pulmonary cryptococcosis mimicking lung cancer. Eur J Cardiothorac Surg 2006;30:837-9. [Crossref] [PubMed]
- Song KD, Lee KS, Chung MP, et al. Pulmonary cryptococcosis: imaging findings in 23 non-AIDS patients. Korean J Radiol 2010;11:407-16. [Crossref] [PubMed]
- Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev 2011;24:247-80. [Crossref] [PubMed]
- Zhang Y, Li N, Zhang Y, et al. Clinical analysis of 76 patients pathologically diagnosed with pulmonary cryptococcosis. Eur Respir J 2012;40:1191-200. [Crossref] [PubMed]
- Fox DL, Müller NL. Pulmonary cryptococcosis in immunocompetent patients: CT findings in 12 patients. AJR Am J Roentgenol 2005;185:622-6. [Crossref] [PubMed]
- Kakeya H, Abe K, Yoshinaga M, et al. Spontaneous resolution of pulmonary cryptococcosis--report of 2 cases. Nihon Kokyuki Gakkai Zasshi 1998;36:902-7. [PubMed]
- Chen CH, Wang CS, Juang YC, et al. Pulmonary cryptococcosis with spontaneous resolution--although with high serum cryptococcal antigen titer. Zhonghua Yi Xue Za Zhi (Taipei) 1988;41:379-82. [PubMed]
- Choi H, Lee H, Jeong SH, et al. Pulmonary actinomycosis mimicking lung cancer on positron emission tomography. Ann Thorac Med 2017;12:121-4. [Crossref] [PubMed]
- Lee H, Yang B, Nam B, et al. Treatment outcomes in patients with extranodal marginal zone B-cell lymphoma of the lung. J Thorac Cardiovasc Surg 2017;154:342-9. [Crossref] [PubMed]
- Haroon J, Paul S. Living on the edge: Does cut mean cure for pulmonary mucosa-associated lymphoid tissue? J Thorac Cardiovasc Surg 2017;154:350-1. [Crossref] [PubMed]
- Fisher JF, Valencia-Rey PA, Davis WB. Pulmonary Cryptococcosis in the Immunocompetent Patient-Many Questions, Some Answers. Open Forum Infect Dis 2016;3:ofw167. [Crossref] [PubMed]